
Acetaldehyde and benzaldehyde can be differentiated by:
A. Fehling’s test
B. Iodoform test
C. Tollens reagent
D. Both ‘A’ and ‘B’
Answer
527.3k+ views
Hint: Take into consideration which reagents are tests for which group, it can be the presence of the aldehyde group, $\alpha $- hydrogen or the acyl group.
Complete step by step answer:
The $\alpha $- hydrogen atoms are the hydrogen atoms that are bonded to the $\alpha $- carbon atoms. $\alpha $- carbon atoms can be identified by their bond to the carbonyl carbon. In aldehydes and ketones, the carbon atoms that are bonded to the carbonyl carbon are called $\alpha $- carbons. In general, the carbon that is directly bonded to a functional group is called the $\alpha $- carbon atom. Further down the line, the carbon atoms directly bonded to the $\alpha $- carbons are the $\beta $- carbons and so on.
First, let us take into consideration the structures on acetaldehyde and benzaldehyde.
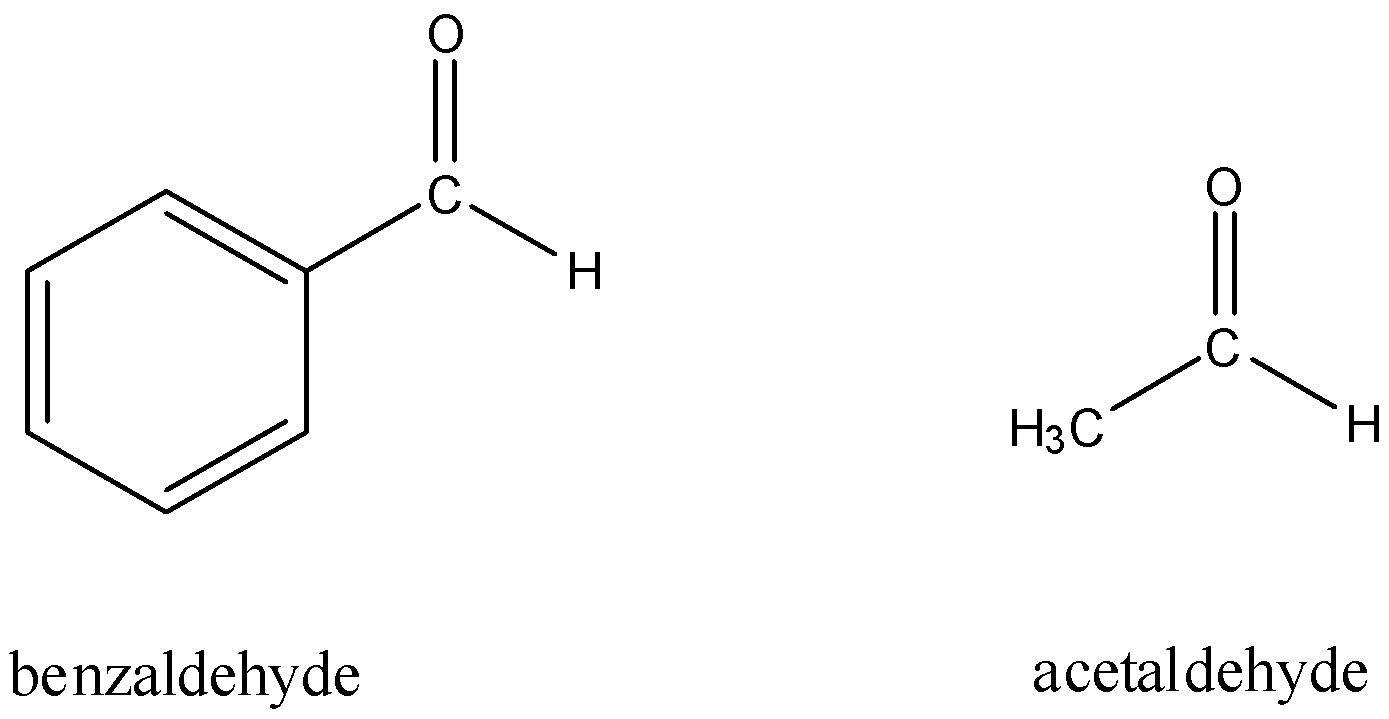
Now, consider Fehling's test.
Fehling’s test gives a reddish-brown precipitate of $Cu{{O}_{2}}$ when it reacts with aldehydes or ketones having an $\alpha $- hydrogen. As we know from the structures of benzaldehyde and acetaldehyde; benzaldehyde has no $\alpha $- hydrogens whereas acetaldehyde has 3 $\alpha $- hydrogens. These $\alpha $- hydrogens facilitate the formation of the enolate complex which is essential for the precipitation of $Cu{{O}_{2}}$. Thus, this test will give a positive result for acetaldehyde and a negative result for benzaldehyde.
Now, consider the Iodoform test.
The iodoform test is used to detect the presence of acyl groups in the given compound. When the test is performed, if a methyl ketone or an acyl group is present in the given compound then a yellow precipitate with an antiseptic smell is formed. This test also gives a positive result if a carbon atom is bonded to a methyl group as well as an alcohol group at the same time. We can see that acetaldehyde has an acyl group whereas benzaldehyde does not. Thus, the test will give a positive result for acetaldehyde and a negative result for benzaldehyde.
Now, consider the Tollens reagent.
Tollens reagent is used to test the presence of aldehydes in a given compound. It is a very strong oxidizing agent and does not depend on the presence of $\alpha $- hydrogens for oxidation and can even oxidize some ketones that may tautomerize into aldehydes. Thus, it can oxidize both acetaldehyde and benzaldehyde and cannot differentiate between them.
Hence, the correct answer is option D.
Note:
Remember that although Fehling’s test and tollens reagents are both usually used to test the presence of aldehydes, it was found that Fehling’s test gives a negative result to aldehydes that do not have an $\alpha $- hydrogen and give a positive result for some $\alpha $- hydroxy ketones. This proves that they test for the presence of $\alpha $- hydrogens and not aldehydes
Complete step by step answer:
The $\alpha $- hydrogen atoms are the hydrogen atoms that are bonded to the $\alpha $- carbon atoms. $\alpha $- carbon atoms can be identified by their bond to the carbonyl carbon. In aldehydes and ketones, the carbon atoms that are bonded to the carbonyl carbon are called $\alpha $- carbons. In general, the carbon that is directly bonded to a functional group is called the $\alpha $- carbon atom. Further down the line, the carbon atoms directly bonded to the $\alpha $- carbons are the $\beta $- carbons and so on.
First, let us take into consideration the structures on acetaldehyde and benzaldehyde.

Now, consider Fehling's test.
Fehling’s test gives a reddish-brown precipitate of $Cu{{O}_{2}}$ when it reacts with aldehydes or ketones having an $\alpha $- hydrogen. As we know from the structures of benzaldehyde and acetaldehyde; benzaldehyde has no $\alpha $- hydrogens whereas acetaldehyde has 3 $\alpha $- hydrogens. These $\alpha $- hydrogens facilitate the formation of the enolate complex which is essential for the precipitation of $Cu{{O}_{2}}$. Thus, this test will give a positive result for acetaldehyde and a negative result for benzaldehyde.
Now, consider the Iodoform test.
The iodoform test is used to detect the presence of acyl groups in the given compound. When the test is performed, if a methyl ketone or an acyl group is present in the given compound then a yellow precipitate with an antiseptic smell is formed. This test also gives a positive result if a carbon atom is bonded to a methyl group as well as an alcohol group at the same time. We can see that acetaldehyde has an acyl group whereas benzaldehyde does not. Thus, the test will give a positive result for acetaldehyde and a negative result for benzaldehyde.
Now, consider the Tollens reagent.
Tollens reagent is used to test the presence of aldehydes in a given compound. It is a very strong oxidizing agent and does not depend on the presence of $\alpha $- hydrogens for oxidation and can even oxidize some ketones that may tautomerize into aldehydes. Thus, it can oxidize both acetaldehyde and benzaldehyde and cannot differentiate between them.
Hence, the correct answer is option D.
Note:
Remember that although Fehling’s test and tollens reagents are both usually used to test the presence of aldehydes, it was found that Fehling’s test gives a negative result to aldehydes that do not have an $\alpha $- hydrogen and give a positive result for some $\alpha $- hydroxy ketones. This proves that they test for the presence of $\alpha $- hydrogens and not aldehydes
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw a labelled sketch of the human eye class 12 physics CBSE

Which are the Top 10 Largest Countries of the World?

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




