
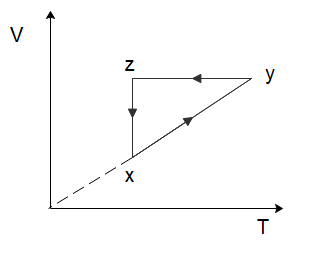
A thermodynamic cycle xyzx is shown on a V−T diagram. The P−V diagram that best describes this cycle is: (Diagrams are schematic and not to scale)
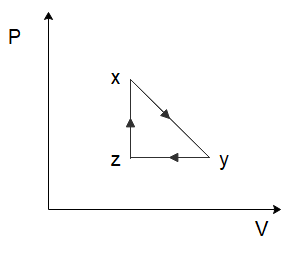
(A)
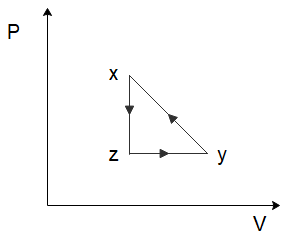
(B)
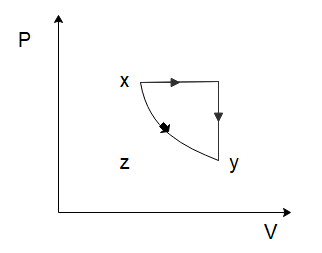
(C)
(D)
Answer
233.1k+ views
Hint: In this solution, we will use the ideal gas formula to determine the relationship between pressure-volume and temperature. For each process, we will determine which quantity remains constant and which quantity changes.
Formula used: In this solution, we will use the following formula:
-Ideal gas law: \[PV = nRT\] where $P$ is the pressure, $V$ is the volume, $n$ is the number of moles, $R$is the gas constant, and $T$ is the temperature
Complete step by step answer:
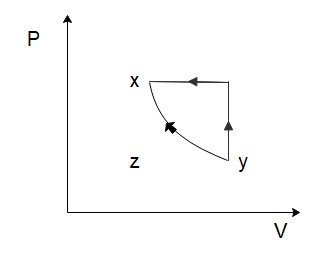
To solve this problem let us start by considering the process of $x \to y$. From the graph, we can see that both the temperature and the volume of the gas are increasing. Now in the relation \[PV = nRT\], $n$ and $R$ are constants. So, if the temperature and the volume both increase, the pressure would have to remain constant for the equation to hold true. So, for the process $x \to y$, the pressure will remain constant while the volume increases. Among the options given to us, this is only shown in option (C).
So the correct option is C
Note: Often, we cannot determine the correct choice from just one process, so let us determine the graphs of the other processes as well.
In the process \[y \to z\], we can see that the volume of the gas remains constant, while the temperature of the gas decreases. Again, from the ideal gas law, we can deduce that the pressure of the gas would have to decrease to ensure the ideal gas law holds true. So, the pressure decreases, and the volume of the gas remains constant which is again only shown in option (C).
Formula used: In this solution, we will use the following formula:
-Ideal gas law: \[PV = nRT\] where $P$ is the pressure, $V$ is the volume, $n$ is the number of moles, $R$is the gas constant, and $T$ is the temperature
Complete step by step answer:
To solve this problem let us start by considering the process of $x \to y$. From the graph, we can see that both the temperature and the volume of the gas are increasing. Now in the relation \[PV = nRT\], $n$ and $R$ are constants. So, if the temperature and the volume both increase, the pressure would have to remain constant for the equation to hold true. So, for the process $x \to y$, the pressure will remain constant while the volume increases. Among the options given to us, this is only shown in option (C).
So the correct option is C
Note: Often, we cannot determine the correct choice from just one process, so let us determine the graphs of the other processes as well.
In the process \[y \to z\], we can see that the volume of the gas remains constant, while the temperature of the gas decreases. Again, from the ideal gas law, we can deduce that the pressure of the gas would have to decrease to ensure the ideal gas law holds true. So, the pressure decreases, and the volume of the gas remains constant which is again only shown in option (C).
Recently Updated Pages
JEE Main 2026 Session 2 Registration Open, Exam Dates, Syllabus & Eligibility

JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

Trending doubts
JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding Average and RMS Value in Electrical Circuits

Understanding Collisions: Types and Examples for Students

Ideal and Non-Ideal Solutions Explained for Class 12 Chemistry

Understanding Atomic Structure for Beginners

Derive an expression for maximum speed of a car on class 11 physics JEE_Main

Other Pages
JEE Advanced Weightage 2025 Chapter-Wise for Physics, Maths and Chemistry

NCERT Solutions For Class 11 Physics Chapter 9 Mechanical Properties of Fluids (2025-26)

NCERT Solutions For Class 11 Physics Chapter 12 Kinetic Theory (2025-26)

NCERT Solutions For Class 11 Physics Chapter 4 Law of Motion (2025-26)

Class 11 JEE Main Physics Mock Test 2025

Inductive Effect and Its Role in Acidic Strength




