
When a mixture of salicylic acid, acetic anhydride and acetic acid is refluxed, what is the product obtained and what is its use in everyday life? Write the equation for the reaction. Why shouldn’t this medicine be taken in an empty stomach?
Answer
573.6k+ views
Hint: Salicylic acid is defined as the beta hydroxy acid which is used for exfoliating skin and reduces acne. It is a colourless crystalline compound which is derived from the metabolism of salicin. Acetic anhydride is a carboxylic anhydride widely used in the reagent in organic synthesis.
Complete step by step answer:
When a mixture of salicylic acid, acetic anhydride and acetic acid is refluxed then the product obtained is also called aspirin. The phenol group present on the salicylic acid forms an ester with carboxyl group on the acetic acid. This reaction is very slow and produces low yield. Aspirin is also known as acetylsalicylic acid.
The reaction is given below:
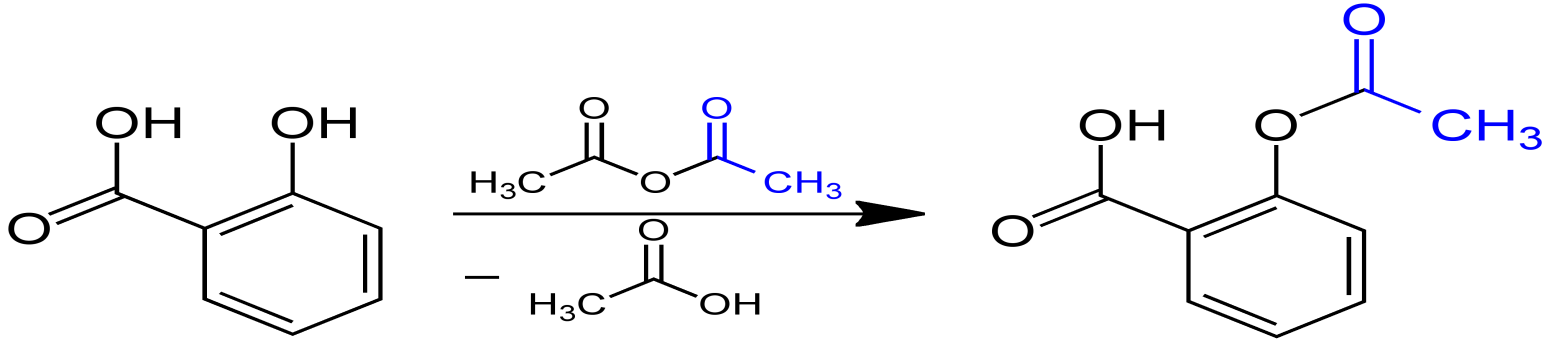
Aspirin is used as an everyday painkiller for aches and pains such as headaches, tooth ache etc. It can also be used to treat cold, cough and fever. It is also used to reduce swelling in diseases like arthritis.
Aspirin is also used for reducing the risk of heart attack. It can be used to treat mild to moderate pain.
Aspirin should not be taken in an empty stomach because it leads to stomach irritation and stomach ache too. It might affect the inner lining of the stomach and can also cause gastric ulcers or bleeding.
Note: Aspirin decomposes rapidly in solutions of ammonium acetate or carbonates. In alkali solutions, the decomposition process proceeds rapidly. Aspirin has been shown to be helpful when used daily to lower the risk of heart attack and other blood flow problems in patients who have cardiovascular disease or who have already had a heart attack.
Complete step by step answer:
When a mixture of salicylic acid, acetic anhydride and acetic acid is refluxed then the product obtained is also called aspirin. The phenol group present on the salicylic acid forms an ester with carboxyl group on the acetic acid. This reaction is very slow and produces low yield. Aspirin is also known as acetylsalicylic acid.
The reaction is given below:

Aspirin is used as an everyday painkiller for aches and pains such as headaches, tooth ache etc. It can also be used to treat cold, cough and fever. It is also used to reduce swelling in diseases like arthritis.
Aspirin is also used for reducing the risk of heart attack. It can be used to treat mild to moderate pain.
Aspirin should not be taken in an empty stomach because it leads to stomach irritation and stomach ache too. It might affect the inner lining of the stomach and can also cause gastric ulcers or bleeding.
Note: Aspirin decomposes rapidly in solutions of ammonium acetate or carbonates. In alkali solutions, the decomposition process proceeds rapidly. Aspirin has been shown to be helpful when used daily to lower the risk of heart attack and other blood flow problems in patients who have cardiovascular disease or who have already had a heart attack.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




