
A mixture of 1-chloropropane and 2-chloropropane when treated with alcoholic $KOH$ gives
A. prop$ - 1 - $ene
B. prop$ - 2 - $ene
C. a mixture of prop$ - 1 - $ene and prop$ - 2 - $ene
D. propanol
Answer
580.8k+ views
Hint: Alkyl halides when treated with alcoholic potassium hydroxide undergo dehydrohalogenation reaction to give alkenes as the product. The alkene is formed by the removal of the hydrogen atom present in the $\beta - C$ atom, along with the removal of chlorine. In other words, the product is an alkene with an $\alpha - \beta $ double bond.
Complete step by step solution:
Alkyl halides tend to form the more substituted alkenes when treated with alcoholic $KOH$. In this reaction, first, the alcohol and the potassium hydroxide split up into their respective ions:
${C_2}{H_5}OH \rightleftharpoons {C_2}{H_5}{O^ - } + {H^ + }$
$KOH \rightleftharpoons {K^ + } + O{H^ - }$
The potassium ion and the ethoxy ion then combines to form potassium ethoxide:
${C_2}{H_5}{O^ - } + {K^ + } \to {C_2}{H_5}OK$
Potassium ethoxide so formed is a very strong base, and like all bases, it tends to attract hydrogen atoms. Thus, this molecule abstracts the $\beta - H$ atom (hydrogen atom present on the carbon atom next to the carbon atom holding the chlorine atom) from both the $1 - $ chloropropane and $2 - $ chloropropane. The chlorine atom then leaves with the hydrogen, and a double bond is formed between the carbon atom which has the chlorine atom, and the carbon atom next to it, thus forming the alkene.
Let us see the exact mechanism of this for $1 - $ chloropropane and $2 - $ chloropropane:
For $1 - $ chloropropane, the reaction is as follows:
$C{H_3}C{H_2}C{H_2}Cl\xrightarrow{{alc.KOH}}C{H_3}CH = C{H_2} + HCl$
Thus, the product formed is prop- $1$ -ene.
For $2 - $ chloropropane, the reaction is as follows:
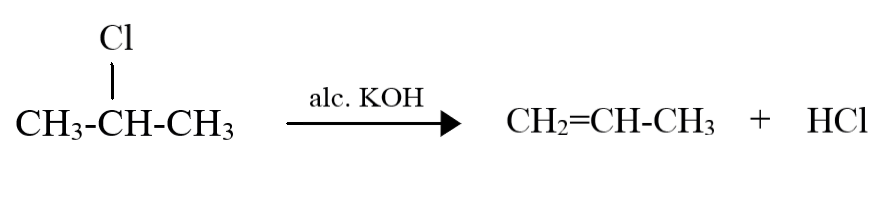
Even in the case of $2 - $ chloropropane, the same product is being formed. This is because as the propane molecule has just three carbon atoms, the carbon atoms on either ends of the molecule are both equally preferred $\beta - C$ atoms, and thus, the product formed will be the same since the double bond can only be formed between the central carbon atom and a carbon atom at either end.
Thus, the products of dehydrohalogenation of $1 - $ chloropropane and $2 - $ chloropropane are the same, and we get prop$ - 1 - $ene as the product.
Hence the option ‘A’ is the correct solution for the given question.
Note:
Since the propane molecule is a symmetrical molecule, it is impossible to have a product named prop$ - 2 - $ene, as IUPAC nomenclature names double bonds according to the first carbon atom in which they are present. Note that the $\beta - H$ atom is abstracted as it is the most acidic hydrogen in the compound, due to the high electronegativity of the halogen.
Complete step by step solution:
Alkyl halides tend to form the more substituted alkenes when treated with alcoholic $KOH$. In this reaction, first, the alcohol and the potassium hydroxide split up into their respective ions:
${C_2}{H_5}OH \rightleftharpoons {C_2}{H_5}{O^ - } + {H^ + }$
$KOH \rightleftharpoons {K^ + } + O{H^ - }$
The potassium ion and the ethoxy ion then combines to form potassium ethoxide:
${C_2}{H_5}{O^ - } + {K^ + } \to {C_2}{H_5}OK$
Potassium ethoxide so formed is a very strong base, and like all bases, it tends to attract hydrogen atoms. Thus, this molecule abstracts the $\beta - H$ atom (hydrogen atom present on the carbon atom next to the carbon atom holding the chlorine atom) from both the $1 - $ chloropropane and $2 - $ chloropropane. The chlorine atom then leaves with the hydrogen, and a double bond is formed between the carbon atom which has the chlorine atom, and the carbon atom next to it, thus forming the alkene.
Let us see the exact mechanism of this for $1 - $ chloropropane and $2 - $ chloropropane:
For $1 - $ chloropropane, the reaction is as follows:
$C{H_3}C{H_2}C{H_2}Cl\xrightarrow{{alc.KOH}}C{H_3}CH = C{H_2} + HCl$
Thus, the product formed is prop- $1$ -ene.
For $2 - $ chloropropane, the reaction is as follows:

Even in the case of $2 - $ chloropropane, the same product is being formed. This is because as the propane molecule has just three carbon atoms, the carbon atoms on either ends of the molecule are both equally preferred $\beta - C$ atoms, and thus, the product formed will be the same since the double bond can only be formed between the central carbon atom and a carbon atom at either end.
Thus, the products of dehydrohalogenation of $1 - $ chloropropane and $2 - $ chloropropane are the same, and we get prop$ - 1 - $ene as the product.
Hence the option ‘A’ is the correct solution for the given question.
Note:
Since the propane molecule is a symmetrical molecule, it is impossible to have a product named prop$ - 2 - $ene, as IUPAC nomenclature names double bonds according to the first carbon atom in which they are present. Note that the $\beta - H$ atom is abstracted as it is the most acidic hydrogen in the compound, due to the high electronegativity of the halogen.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE

Explain sex determination in humans with line diag class 12 biology CBSE

Organisms of a higher trophic level which feed on several class 12 biology CBSE




