
A compound (X) reacts with thionyl chloride to give a compound (Y). (Y) Reacts with $Mg$ to form a Grignard reagent, which is treated with acetone and the product is hydrolysed to give $2 - methyl - 2 - butanol$. What are the structural formulae of (X) and (Y)?
A. X= Acetic acid Y= Ethyl chloride
B. X=Acetaldehyde Y= Ethyl chloride
C. X=Ethyl Alcohol Y=Ethyl chloride
D. X=Propyl Alcohol Y=Propyl chloride
Answer
573.9k+ views
Hint:The final compound contains five carbon atoms, and it was produced from acetone, which has three carbon atoms. Therefore, the Grignard reagent would have two carbon atoms. Since Y is the compound from which the Grignard reagent is formed, it must be an alkyl chloride, indicating that compound X is an alcohol.
Complete step by step answer:
We will work from behind to find the solution to this question. The structure of the final product is given below:
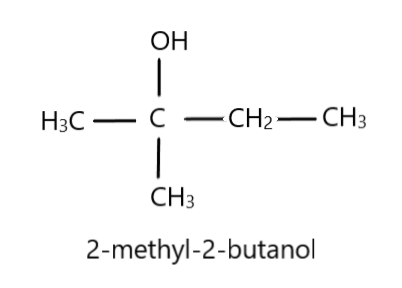
Since it contains five carbon atoms and acetone has three, we will get the number of carbon atoms in the Grignard reagent by just subtracting the number of carbon atoms in the product and acetone. Therefore, the Grignard reagent should have two carbon atoms. This is because in the reaction of Grignard reagent and acetone, the keto group gets reduced to alcohol, and the alkyl part of the Grignard reagent gets attached to the central carbon atom of acetone. And since thionyl chloride was used to produce Y, the halogen in the Grignard reagent would be chlorine. Hence, the Grignard reagent would be $C{H_3}C{H_2}MgCl$ .
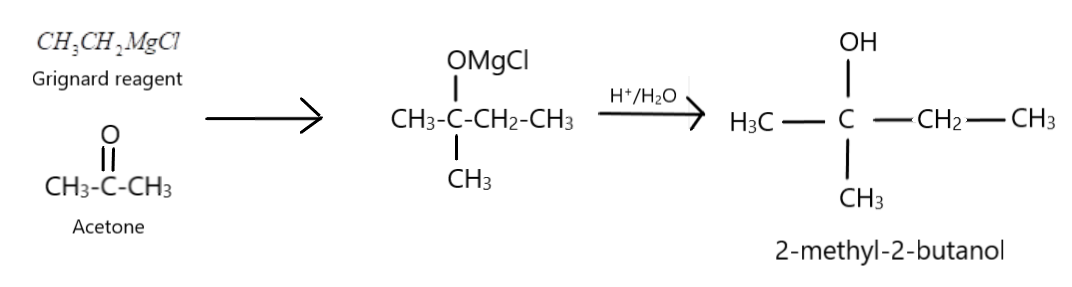
Since compound Y reacts with $Mg$ to give the above Grignard reagent, Y would be the corresponding alkyl chloride: $C{H_3}C{H_2}Cl$
$C{H_3}C{H_2}Cl + Mg \to C{H_3}C{H_2}MgCl$
(Y)
Thus, compound X reacts with thionyl chloride ( $SOC{l_2}$ ) to give $C{H_3}C{H_2}Cl$ , indicating that compound X is the corresponding alcohol, since, alcohols react with thionyl chloride to give alkyl chlorides. Therefore, X is $C{H_3}C{H_2}OH$ .
$C{H_3}C{H_2}OH + SOC{l_2} \to C{H_3}C{H_2}Cl + HCl + S{O_2}$
(X)
Hence, X is ethyl alcohol and Y is ethyl chloride.
Hence, the correct option is $C$ .
Note:
The sulphur dioxide produced when thionyl chloride reacts with alcohols escapes a gas, and thus, this is a characteristic test for organic alcohols. Also note that Grignard reagents are commonly used as reducing agents, to convert ketones and aldehydes into their corresponding alcohols. Note that ketones always give a tertiary alcohol when reduced with a Grignard reagent.
Complete step by step answer:
We will work from behind to find the solution to this question. The structure of the final product is given below:

Since it contains five carbon atoms and acetone has three, we will get the number of carbon atoms in the Grignard reagent by just subtracting the number of carbon atoms in the product and acetone. Therefore, the Grignard reagent should have two carbon atoms. This is because in the reaction of Grignard reagent and acetone, the keto group gets reduced to alcohol, and the alkyl part of the Grignard reagent gets attached to the central carbon atom of acetone. And since thionyl chloride was used to produce Y, the halogen in the Grignard reagent would be chlorine. Hence, the Grignard reagent would be $C{H_3}C{H_2}MgCl$ .

Since compound Y reacts with $Mg$ to give the above Grignard reagent, Y would be the corresponding alkyl chloride: $C{H_3}C{H_2}Cl$
$C{H_3}C{H_2}Cl + Mg \to C{H_3}C{H_2}MgCl$
(Y)
Thus, compound X reacts with thionyl chloride ( $SOC{l_2}$ ) to give $C{H_3}C{H_2}Cl$ , indicating that compound X is the corresponding alcohol, since, alcohols react with thionyl chloride to give alkyl chlorides. Therefore, X is $C{H_3}C{H_2}OH$ .
$C{H_3}C{H_2}OH + SOC{l_2} \to C{H_3}C{H_2}Cl + HCl + S{O_2}$
(X)
Hence, X is ethyl alcohol and Y is ethyl chloride.
Hence, the correct option is $C$ .
Note:
The sulphur dioxide produced when thionyl chloride reacts with alcohols escapes a gas, and thus, this is a characteristic test for organic alcohols. Also note that Grignard reagents are commonly used as reducing agents, to convert ketones and aldehydes into their corresponding alcohols. Note that ketones always give a tertiary alcohol when reduced with a Grignard reagent.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




