
How is 1-propoxypropane synthesized from propan-1-ol? Write mechanism of this reaction.
Answer
582.9k+ views
Hint: The synthesis takes place by the action of the protic acids such as phosphoric acid or Sulphuric acid and there is a loss of water molecule for the formation of product.
Complete step by step answer:
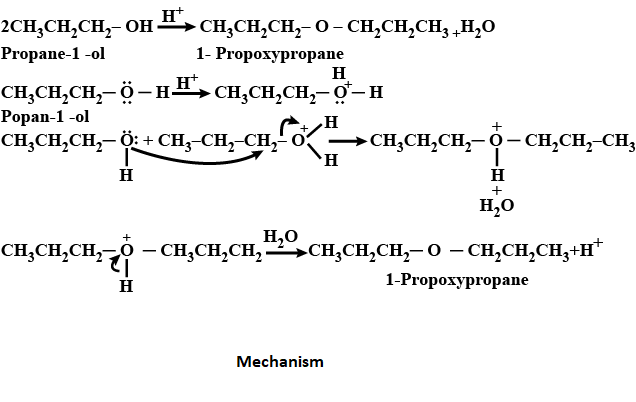
1-propoxypropane is synthesized from propan-1-ol by the actions of the protic acids such as Sulphuric acid or phosphoric acid etc. Two molecules of propan-1-ol condense to form 1 molecule of 1-propoxypropane. During this process one molecule of water is lost.
\[2C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}-OH\xrightarrow{{{H}^{+}}}C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}-O-C{{H}_{2}}C{{H}_{2}}C{{H}_{3}}\]
Dehydration is the conversion that involves the loss of water molecules from the reacting molecule.
Protonation is the addition of a proton to an atom or molecule or ion forming a conjugate acid.
Deprotonation is the process of the removal of a proton from an acid in the acid-base reaction.
The reaction mechanism is explained below:
First step: protonation- The first step involves the protonation of the oxygen atom present in propan-1-ol.
Second step: nucleophile attack- in this step the oxygen atom of the second molecule of propan-1-ol which acts as a nucleophile here attacks the protonated form of propan-1-ol and forms protonated 1-propoxypropane and water.
Third step: deprotonation- in this step deprotonating of protonated 1-propoxypropane takes place.
Note: 1-propanol is used as a solvent as a multi-purpose solvent in the industry and in the home. It is used in many of the textile applications and also used in cosmetic products and lotions, in window cleaning, polishing etc. There is a toxic effect of 1-propanol, it causes depression of the central nervous system 1-propanol is neurotoxic.
Complete step by step answer:
1-propoxypropane is synthesized from propan-1-ol by the actions of the protic acids such as Sulphuric acid or phosphoric acid etc. Two molecules of propan-1-ol condense to form 1 molecule of 1-propoxypropane. During this process one molecule of water is lost.
\[2C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}-OH\xrightarrow{{{H}^{+}}}C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}-O-C{{H}_{2}}C{{H}_{2}}C{{H}_{3}}\]
Dehydration is the conversion that involves the loss of water molecules from the reacting molecule.
Protonation is the addition of a proton to an atom or molecule or ion forming a conjugate acid.
Deprotonation is the process of the removal of a proton from an acid in the acid-base reaction.
The reaction mechanism is explained below:
First step: protonation- The first step involves the protonation of the oxygen atom present in propan-1-ol.
Second step: nucleophile attack- in this step the oxygen atom of the second molecule of propan-1-ol which acts as a nucleophile here attacks the protonated form of propan-1-ol and forms protonated 1-propoxypropane and water.
Third step: deprotonation- in this step deprotonating of protonated 1-propoxypropane takes place.

Note: 1-propanol is used as a solvent as a multi-purpose solvent in the industry and in the home. It is used in many of the textile applications and also used in cosmetic products and lotions, in window cleaning, polishing etc. There is a toxic effect of 1-propanol, it causes depression of the central nervous system 1-propanol is neurotoxic.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




