
Which one of the following statements is not true?
1) Lactose contains \[\alpha \] -glycosidic linkage between \[{C_1}\] linkage of galactose and \[{C_4}\] of glucose.
2) Lactose is a reducing sugar and gives Fehling’s test.
3) On acid hydrolysis, lactose gives one molecule of D(+)-glucose and one molecule of D(+)- galactose.
4) Lactose (\[{{\rm{C}}_{{\rm{11}}}}{{\rm{H}}_{{\rm{22}}}}{{\rm{O}}_{{\rm{11}}}}\] ) is a disaccharide and it contains 8 hydroxyl groups.
Answer
233.1k+ views
Hint: When two monosaccharides or simple sugars undergo combination, the formation of disaccharides occurs. Some names of disaccharides are maltose, sucrose, lactose, etc. It is found in various milk forms.
Complete Step by Step Answer:
Let's discuss all the options one by one.
The structure of lactose is,
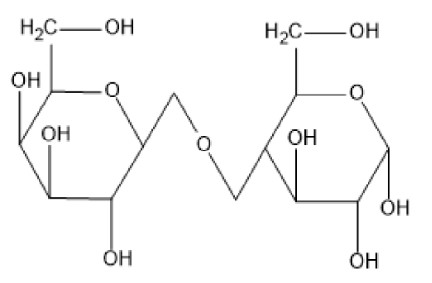
Fig: Lactose
The linkage between the \[{C_1}\] linkage of galactose and \[{C_4}\] of glucose is \[\beta \] -glycosidic linkage. Therefore, statement 1 is false.
The reducing sugars are those which behaves as a reducing agent in a solution of alkaline nature is termed reducing sugar. Lactose is a reducing sugar. Fehling's test is useful for the differentiation of ketone and aldehyde. And lactose gives a positive Fehling's test. Therefore, statement 2 is true.
Hydrolysis is the removal of a water molecule from a compound. On acidic hydrolysis of lactose, a molecule of D(+)- galactose and a molecule of D(+)-glucose each are obtained. Therefore, statement 3 is true. From the above diagram of glucose, we see that there are eight numbers of hydroxyl groups in the molecule of glucose and it is a disaccharide. Therefore, statement 4 is true.
Therefore, option 1 is right.
Note: Lactose intolerance is the condition in which our body is not capable of digesting lactose found in milk and other milk products. This occurs because of the shortage of adequate lactase which is produced by the small intestine and this enzyme helps in the digestion of lactose.
Complete Step by Step Answer:
Let's discuss all the options one by one.
The structure of lactose is,

Fig: Lactose
The linkage between the \[{C_1}\] linkage of galactose and \[{C_4}\] of glucose is \[\beta \] -glycosidic linkage. Therefore, statement 1 is false.
The reducing sugars are those which behaves as a reducing agent in a solution of alkaline nature is termed reducing sugar. Lactose is a reducing sugar. Fehling's test is useful for the differentiation of ketone and aldehyde. And lactose gives a positive Fehling's test. Therefore, statement 2 is true.
Hydrolysis is the removal of a water molecule from a compound. On acidic hydrolysis of lactose, a molecule of D(+)- galactose and a molecule of D(+)-glucose each are obtained. Therefore, statement 3 is true. From the above diagram of glucose, we see that there are eight numbers of hydroxyl groups in the molecule of glucose and it is a disaccharide. Therefore, statement 4 is true.
Therefore, option 1 is right.
Note: Lactose intolerance is the condition in which our body is not capable of digesting lactose found in milk and other milk products. This occurs because of the shortage of adequate lactase which is produced by the small intestine and this enzyme helps in the digestion of lactose.
Recently Updated Pages
JEE Main 2026 Session 2 Registration Open, Exam Dates, Syllabus & Eligibility

JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

Trending doubts
JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding Average and RMS Value in Electrical Circuits

Understanding Collisions: Types and Examples for Students

Ideal and Non-Ideal Solutions Explained for Class 12 Chemistry

Understanding Atomic Structure for Beginners

AssertionIn electrolytic refining of metal impure metal class 12 chemistry JEE_Main

Other Pages
JEE Advanced Weightage 2025 Chapter-Wise for Physics, Maths and Chemistry

NCERT Solutions For Class 12 Chemistry Chapter 2 Solutions Hindi Medium (2025-26)

CBSE Class 12 Chemistry Set 1 56/2/1 2025: Question Paper, Answers & Analysis

CBSE Class 12 Chemistry Question Paper Set 3 2025 with Answers

Inductive Effect and Its Role in Acidic Strength

Degree of Dissociation: Meaning, Formula, Calculation & Uses




