
Which one of the following molecules will have unequal M−F bond lengths?
A.\[{\rm{N}}{{\rm{F}}_{\rm{3}}}\]
B. \[{\rm{B}}{{\rm{F}}_{\rm{3}}}\]
C. \[{\rm{P}}{{\rm{F}}_{\rm{5}}}\]
D. None of these
Answer
233.4k+ views
Hint: Bond length is the length between the nuclei of two atoms in a bond pair in a molecule. Its units are Angstrom (\[{10^{ - 10}}\] m) or picometer (\[{10^{ - 12}}\] m). The presence of unequal M−F bond lengths will be determined by the shape of molecules.
Complete Step by Step Solution:
The shape of molecules is depicted by Valence Shell Electron Pair Repulsion (VSEPR) Theory.
It is established on the repulsive relation between the electron pairs in the valence shell of the atoms.
This theory was furnished by Sidgwick and Powell in 1940 and was later enhanced by Nyholm and Gillespie in 1957.
The major postulates of this theory are as comes next:-
The shape of the molecule relies upon the no.of valence shell bonded or non-bonded electron pairs around the central atom.
Pairs of electrons in the valence shell repulse one another as they both have negative charges.
These pairs of electrons manage to settle in a region that decreases repulsion and therefore the distance between them increases.
The valence shell is assumed as a sphere with the electron pairs on the spherical surface at the utmost space from one another.
Lone pair-lone pair repulsion is the highest. It is followed by lone pair-bond pair repulsion and bond pair-bond pair repulsion.
We have to find out which of the following molecules will have unequal M-F bond lengths.
A. \[{\rm{N}}{{\rm{F}}_{\rm{3}}}\]
Here nitrogen is the central atom. It possesses five electrons in the valence shell.
So, there will be three bond pairs and one lone pair.
The expected shape is tetrahedral.
Due to lone pair-bond pair repulsion, the shape will be trigonal pyramidal.
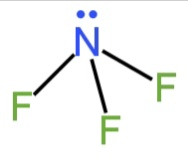
Image: Structure of \[{\rm{N}}{{\rm{F}}_{\rm{3}}}\].
It does not contain unequal M−F bond length as the lone pair-bond pair repulsion is not that strong.
So, A is incorrect.
B. \[{\rm{B}}{{\rm{F}}_{\rm{3}}}\]
Here boron is the central atom. It possesses three electrons in the valence shell.
So, there will be three bond pairs.
The shape is trigonal planar.
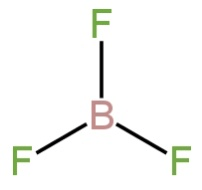
Image: Structure of \[{\rm{B}}{{\rm{F}}_{\rm{3}}}\].
It does not contain unequal M−F bond length as no repulsion is there.
So, B is incorrect.
C. \[{\rm{P}}{{\rm{F}}_{\rm{5}}}\]
Here phosphorus is the central atom. It possesses five electrons in the valence shell.
So, there will be three bond pairs.
The shape is trigonal bipyramidal.
Due to bond pair-bond pair repulsion, the M-F bond lengths are different.
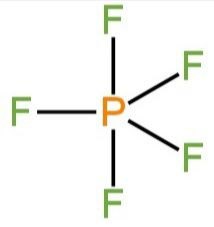
Image: Structure of \[{\rm{P}}{{\rm{F}}_{\rm{5}}}\]
Two axial P-F bonds remain along the axis.
Three equatorial bonds remain in the equatorial position.
The two axial bonds and three equatorial bonds repel each other.
So, axial bonds are longer (240 pm) than equatorial bonds(202 pm).
So, C is correct.
So, option C is correct.
Note: According to the Valence Shell Electron Pair Repulsion (VSEPR) Theory, pairs of electrons in the valence shell repulse one another as like-charges repel each other. So, these pairs of electrons try to remain far away from each other to decrease repulsion. In \[{\rm{P}}{{\rm{F}}_{\rm{5}}}\], there are five bond pairs. The bonded electron pairs repel each other. So, to avoid the repulsion between bond pairs, axial bonds are longer than equatorial bonds in \[{\rm{P}}{{\rm{F}}_{\rm{5}}}\].
Complete Step by Step Solution:
The shape of molecules is depicted by Valence Shell Electron Pair Repulsion (VSEPR) Theory.
It is established on the repulsive relation between the electron pairs in the valence shell of the atoms.
This theory was furnished by Sidgwick and Powell in 1940 and was later enhanced by Nyholm and Gillespie in 1957.
The major postulates of this theory are as comes next:-
The shape of the molecule relies upon the no.of valence shell bonded or non-bonded electron pairs around the central atom.
Pairs of electrons in the valence shell repulse one another as they both have negative charges.
These pairs of electrons manage to settle in a region that decreases repulsion and therefore the distance between them increases.
The valence shell is assumed as a sphere with the electron pairs on the spherical surface at the utmost space from one another.
Lone pair-lone pair repulsion is the highest. It is followed by lone pair-bond pair repulsion and bond pair-bond pair repulsion.
We have to find out which of the following molecules will have unequal M-F bond lengths.
A. \[{\rm{N}}{{\rm{F}}_{\rm{3}}}\]
Here nitrogen is the central atom. It possesses five electrons in the valence shell.
So, there will be three bond pairs and one lone pair.
The expected shape is tetrahedral.
Due to lone pair-bond pair repulsion, the shape will be trigonal pyramidal.

Image: Structure of \[{\rm{N}}{{\rm{F}}_{\rm{3}}}\].
It does not contain unequal M−F bond length as the lone pair-bond pair repulsion is not that strong.
So, A is incorrect.
B. \[{\rm{B}}{{\rm{F}}_{\rm{3}}}\]
Here boron is the central atom. It possesses three electrons in the valence shell.
So, there will be three bond pairs.
The shape is trigonal planar.

Image: Structure of \[{\rm{B}}{{\rm{F}}_{\rm{3}}}\].
It does not contain unequal M−F bond length as no repulsion is there.
So, B is incorrect.
C. \[{\rm{P}}{{\rm{F}}_{\rm{5}}}\]
Here phosphorus is the central atom. It possesses five electrons in the valence shell.
So, there will be three bond pairs.
The shape is trigonal bipyramidal.
Due to bond pair-bond pair repulsion, the M-F bond lengths are different.

Image: Structure of \[{\rm{P}}{{\rm{F}}_{\rm{5}}}\]
Two axial P-F bonds remain along the axis.
Three equatorial bonds remain in the equatorial position.
The two axial bonds and three equatorial bonds repel each other.
So, axial bonds are longer (240 pm) than equatorial bonds(202 pm).
So, C is correct.
So, option C is correct.
Note: According to the Valence Shell Electron Pair Repulsion (VSEPR) Theory, pairs of electrons in the valence shell repulse one another as like-charges repel each other. So, these pairs of electrons try to remain far away from each other to decrease repulsion. In \[{\rm{P}}{{\rm{F}}_{\rm{5}}}\], there are five bond pairs. The bonded electron pairs repel each other. So, to avoid the repulsion between bond pairs, axial bonds are longer than equatorial bonds in \[{\rm{P}}{{\rm{F}}_{\rm{5}}}\].
Recently Updated Pages
JEE Main 2026 Session 2 Registration Open, Exam Dates, Syllabus & Eligibility

JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

Trending doubts
JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding Average and RMS Value in Electrical Circuits

Understanding Collisions: Types and Examples for Students

Ideal and Non-Ideal Solutions Explained for Class 12 Chemistry

Understanding Atomic Structure for Beginners

AssertionIn electrolytic refining of metal impure metal class 12 chemistry JEE_Main

Other Pages
JEE Advanced Weightage 2025 Chapter-Wise for Physics, Maths and Chemistry

NCERT Solutions For Class 12 Chemistry Chapter 2 Solutions Hindi Medium (2025-26)

CBSE Class 12 Chemistry Set 1 56/2/1 2025: Question Paper, Answers & Analysis

CBSE Class 12 Chemistry Question Paper Set 3 2025 with Answers

Inductive Effect and Its Role in Acidic Strength

Degree of Dissociation: Meaning, Formula, Calculation & Uses




