
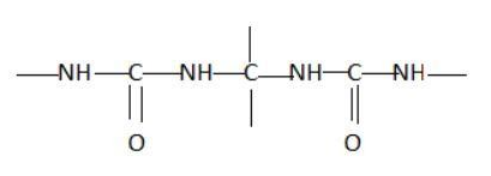
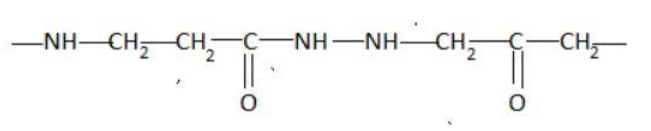
Which of the following represents a peptide chain?
A.
B.
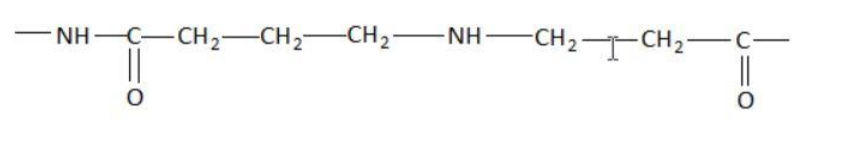
C.
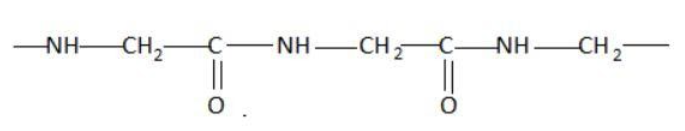
D.
Answer
232.8k+ views
Hint: A peptide chain is formed by combination of amino acids. An amino acid contains carbonyl and amine functional groups with a side chain specific to each amino group. We can detect a peptide link by looking for 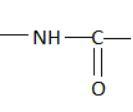 group in the chain.
group in the chain.
Two amino acids combine to form a dipeptide which further goes on linking to form a peptide chain.
Complete step by step answer:
1: Threshold Energy is the minimum Kinetic Energy the molecules must have to bring about effective collisions between two reactant molecules which are considered as spheres, thus resulting in a chemical reaction between the two reactants.
2: Thus, we can write:
Threshold Energy = Average of the initial Kinetic Energy of reactants + Activation Energy (${E_a}$)
3: In case the reactants at the initial state (start of the reaction) have sufficient energy for the collisions, then the threshold energy is equal to Activation Energy. (${E_a}$)
4: Molecules that have a Kinetic Energy equal to or higher than the Threshold Energy will react.
Note:
The cut-off number of amino acids for peptides is traditionally taken between 2 and 50 amino acids, while for proteins it is 50 or more.
Peptide bond is a type of covalent chemical bond linking two consecutive alpha-amino acids from C1 of one amino acid to N2 of the other. Peptide bond is one of the strongest and most durable covalent bond. It can be broken in the laboratory using heat and acid.
 group in the chain.
group in the chain.Two amino acids combine to form a dipeptide which further goes on linking to form a peptide chain.
Complete step by step answer:
1: Threshold Energy is the minimum Kinetic Energy the molecules must have to bring about effective collisions between two reactant molecules which are considered as spheres, thus resulting in a chemical reaction between the two reactants.
2: Thus, we can write:
Threshold Energy = Average of the initial Kinetic Energy of reactants + Activation Energy (${E_a}$)
3: In case the reactants at the initial state (start of the reaction) have sufficient energy for the collisions, then the threshold energy is equal to Activation Energy. (${E_a}$)
4: Molecules that have a Kinetic Energy equal to or higher than the Threshold Energy will react.
Note:
The cut-off number of amino acids for peptides is traditionally taken between 2 and 50 amino acids, while for proteins it is 50 or more.
Peptide bond is a type of covalent chemical bond linking two consecutive alpha-amino acids from C1 of one amino acid to N2 of the other. Peptide bond is one of the strongest and most durable covalent bond. It can be broken in the laboratory using heat and acid.
Recently Updated Pages
Types of Solutions in Chemistry: Explained Simply

JEE General Topics in Chemistry Important Concepts and Tips

JEE Extractive Metallurgy Important Concepts and Tips for Exam Preparation

JEE Amino Acids and Peptides Important Concepts and Tips for Exam Preparation

JEE Atomic Structure and Chemical Bonding important Concepts and Tips

Electricity and Magnetism Explained: Key Concepts & Applications

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




