
Which of the following is the formula of tartar emetic?
A. 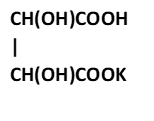
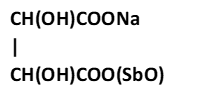
B.

C.
D.
Answer
233.4k+ views
Hint: Tartaric acid is an organic acid that occurs naturally in so many fruits like grapes, bananas, tamarinds, etc. Its chemical formula is HO2CCH(OH)CH(OH)CO2H. The salts of tartaric acids are called cream of tartar, which forms during the process of fermentation.
Complete step by step solution:Tartaric acid is an organic acid and is a derivative of succinic acid. It contains two hydroxy groups and two carboxylic acid groups. There are several derivatives of tartaric acid like the salt of tartaric acid, cream of tartar (potassium bitartrate), Rochelle salt (potassium sodium tartrate), and tartar emetic (antimony potassium tartrate).
Structures of most common salts of tartaric acid-
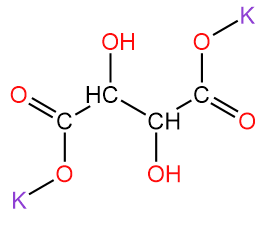
1. Potassium tartrate/ dipotassium tartrate/ argol with chemical formula K2C4H4O6.
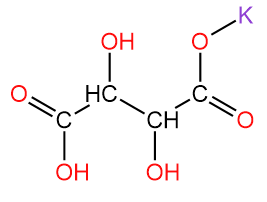
2. Potassium bitartrate/ potassium hydrogen tartrate/ cream of tartar with chemical formula KC4H5O6.
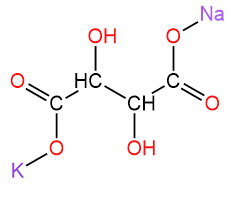
3. Potassium sodium tartrate tetrahydrate/ Rochelle Salt/ is a double salt with the chemical formula KNaC4H4O6.
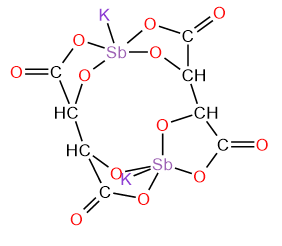
4. Antimony potassium tartrate/ tartar emetic with chemical formula K2Sb2(C4H2O6)2.
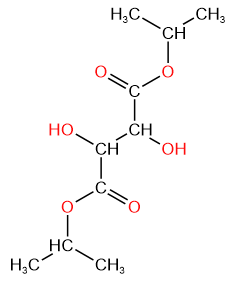
5. Diisopropyl tartrate is a diester of tartaric acid with the chemical formula C10H18O6.
So, these are some examples of salts of tartaric acid and we can see that Antimony potassium tartrate is called tartar emetic. So, all the above answers are incorrect but since tartar emetic can be prepared by heating potassium tartrate with antimony oxide. Hence, the correct answer is Potassium Tartrate.
Thus, Option (C) is correct
Note: Antimony potassium tartrate is called tartar emetic because emetic means the medicine or any substance that causes the feeling of nausea and vomiting. It is toxic in nature but it is used to have an emetic effect and consumed in small quantities with wine.
Complete step by step solution:Tartaric acid is an organic acid and is a derivative of succinic acid. It contains two hydroxy groups and two carboxylic acid groups. There are several derivatives of tartaric acid like the salt of tartaric acid, cream of tartar (potassium bitartrate), Rochelle salt (potassium sodium tartrate), and tartar emetic (antimony potassium tartrate).
Structures of most common salts of tartaric acid-
1. Potassium tartrate/ dipotassium tartrate/ argol with chemical formula K2C4H4O6.

2. Potassium bitartrate/ potassium hydrogen tartrate/ cream of tartar with chemical formula KC4H5O6.

3. Potassium sodium tartrate tetrahydrate/ Rochelle Salt/ is a double salt with the chemical formula KNaC4H4O6.

4. Antimony potassium tartrate/ tartar emetic with chemical formula K2Sb2(C4H2O6)2.

5. Diisopropyl tartrate is a diester of tartaric acid with the chemical formula C10H18O6.

So, these are some examples of salts of tartaric acid and we can see that Antimony potassium tartrate is called tartar emetic. So, all the above answers are incorrect but since tartar emetic can be prepared by heating potassium tartrate with antimony oxide. Hence, the correct answer is Potassium Tartrate.
Thus, Option (C) is correct
Note: Antimony potassium tartrate is called tartar emetic because emetic means the medicine or any substance that causes the feeling of nausea and vomiting. It is toxic in nature but it is used to have an emetic effect and consumed in small quantities with wine.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




