
Which of the following is not an electromagnetic wave?
A) Light Rays
B) X-Rays
C) Alpha Rays
D) Gamma Rays
Answer
232.8k+ views
Hint: Remember the electromagnetic areas and aim to deduce which of the above alternatives does not belong to the spectrum. Think on how and when the rays appear as they are released.
Complete step by step solution:
Electromagnetic waves are also called EM waves, which come into contact with the magnetic field as the electric field comes into contact. The composition of oscillating electrical and magnetic fields may also be assumed to be electromagnetic waves. Electromagnetic waves are solutions to Maxwell's equations, which are the simplest electrodynamics equations.
Electromagnetic waves can be categorised by their varying wavelengths / frequencies and arranged as an electromagnetic continuum. The table below tells us this set consists of some kind of electromagnetic radiation in our world.
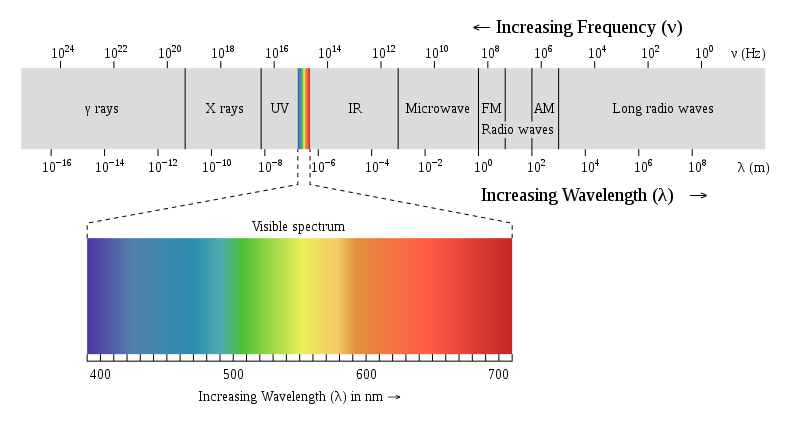
If we can see, only a small percentage of the various forms of radiation exist within the visible range, that is, light that is visible through the eyes. In the right side of the visible spectrum, the sources of radiation are less commonly (and thus more frequently in wavelength) than visible light. We include UV, X-rays and gamma-rays on the left half of the visible spectrum. Owing to their incredibly high frequencies, these forms of radiation damage living species.
The waveform is better viewed as random for some types of EM waves and then a spectral analysis has to be conducted using somewhat different statistical techniques suited to the random or stochastic processes. In such cases, their power content is represented by each frequency variable and phase information is not retained. The power spectral density of a random process is considered such a representation.
The correct option is (C).
Note: The behaviour and relationship of EM radiation to matter is dependent on its frequency and qualitatively varies as the frequency increases. Lower frequencies have longer wavelengths, higher frequencies have shorter wavelengths, and photons of higher energies are associated with them.
Complete step by step solution:
Electromagnetic waves are also called EM waves, which come into contact with the magnetic field as the electric field comes into contact. The composition of oscillating electrical and magnetic fields may also be assumed to be electromagnetic waves. Electromagnetic waves are solutions to Maxwell's equations, which are the simplest electrodynamics equations.
Electromagnetic waves can be categorised by their varying wavelengths / frequencies and arranged as an electromagnetic continuum. The table below tells us this set consists of some kind of electromagnetic radiation in our world.

If we can see, only a small percentage of the various forms of radiation exist within the visible range, that is, light that is visible through the eyes. In the right side of the visible spectrum, the sources of radiation are less commonly (and thus more frequently in wavelength) than visible light. We include UV, X-rays and gamma-rays on the left half of the visible spectrum. Owing to their incredibly high frequencies, these forms of radiation damage living species.
The waveform is better viewed as random for some types of EM waves and then a spectral analysis has to be conducted using somewhat different statistical techniques suited to the random or stochastic processes. In such cases, their power content is represented by each frequency variable and phase information is not retained. The power spectral density of a random process is considered such a representation.
The correct option is (C).
Note: The behaviour and relationship of EM radiation to matter is dependent on its frequency and qualitatively varies as the frequency increases. Lower frequencies have longer wavelengths, higher frequencies have shorter wavelengths, and photons of higher energies are associated with them.
Recently Updated Pages
Circuit Switching vs Packet Switching: Key Differences Explained

JEE General Topics in Chemistry Important Concepts and Tips

JEE Extractive Metallurgy Important Concepts and Tips for Exam Preparation

JEE Amino Acids and Peptides Important Concepts and Tips for Exam Preparation

JEE Atomic Structure and Chemical Bonding important Concepts and Tips

Electricity and Magnetism Explained: Key Concepts & Applications

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Dual Nature of Radiation and Matter Class 12 Physics Chapter 11 CBSE Notes - 2025-26

Understanding Uniform Acceleration in Physics

Understanding the Electric Field of a Uniformly Charged Ring

JEE Advanced Weightage 2025 Chapter-Wise for Physics, Maths and Chemistry

Derivation of Equation of Trajectory Explained for Students




