
Which of the following is not a nitro derivative?
(a) \[{C_6}{H_5}N{O_2}\]
(b) \[C{H_3}C{H_2}ONO\]
(c) \[{(C{H_3})_2}CHN{O_2}\]
(d) \[{C_6}{H_4}(OH)N{O_2}\]
Answer
233.1k+ views
Hint: In nitro ethane (\[{C_2}{H_5}N{O_2}\]) the nitrogen atom is directly attached to the carbon atom of ethane. Whereas in ethyl nitrite (\[{C_2}{H_5}ON{O_2}\]) there is no direct nitrogen, and a carbon bond is present.
Complete step-by-step answer:
The nitro alkanes (\[R - N{O_2}\]) are the organic compounds that are called derivatives of alkanes.
The Presence of nitro group (\[ - N{O_2}\]) in nitro alkanes makes the \[C - H\] protons acidic i.e., \[ - N{O_2}\]strongly considered electron-withdrawing in nature.
The nitro groups are also known as explosophores i.e., they are present in various explosives. For example, Trinitrotoluene or TNT.
In nitro alkane, the \[ - N{O_2}\] group can be bonded to the alkane in two different ways. Therefore, it is called the ambident group.
When the nitrogen atom of the\[ - N{O_2}\]group is attached to the carbon atom of alkane, then it is called nitroalkane i.e., \[R - N{O_2}\].
While in alkane nitrite (\[R - ONO\]), there is no direct nitrogen and carbon bond present. On the other hand, their carbon atom is linked to the nitrogen atom via oxygen atoms.
The alkane nitrites are considered an isomeric form of nitroalkane.
The alkyl nitrites are the esters of nitrous acid.
The nitro alkane can be classified into three classes depending upon the nature of carbon bonded to nitrogen atom:
Primary nitro alkane
When there is methane, or a normal alkyl chain present then it is called primary nitro alkane.

Image: general structure of primary nitro alkane.
Secondary nitro alkane
When the isopropyl group is attached to the nitro group it is called secondary nitro alkane.
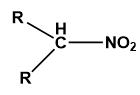
Image: General structure of secondary nitro alkane.
Tertiary nitroalkane
When the tertiary carbon atom is fused with the nitro group then it is called tertiary nitroalkane.
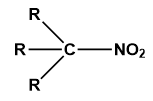
Image: General structure of tertiary nitroalkane.
Hence, from the above discussion. Option (b) will be the correct answer because \[C{H_3}C{H_2}ONO\] does not contain direct nitrogen and carbon bonds.
Note: The organic compounds which contain the nitro group are acidic in nature. Nitro compounds are used in the manufacturing of detergents, dyes, pharmaceuticals, and explosives.
Complete step-by-step answer:
The nitro alkanes (\[R - N{O_2}\]) are the organic compounds that are called derivatives of alkanes.
The Presence of nitro group (\[ - N{O_2}\]) in nitro alkanes makes the \[C - H\] protons acidic i.e., \[ - N{O_2}\]strongly considered electron-withdrawing in nature.
The nitro groups are also known as explosophores i.e., they are present in various explosives. For example, Trinitrotoluene or TNT.
In nitro alkane, the \[ - N{O_2}\] group can be bonded to the alkane in two different ways. Therefore, it is called the ambident group.
When the nitrogen atom of the\[ - N{O_2}\]group is attached to the carbon atom of alkane, then it is called nitroalkane i.e., \[R - N{O_2}\].
While in alkane nitrite (\[R - ONO\]), there is no direct nitrogen and carbon bond present. On the other hand, their carbon atom is linked to the nitrogen atom via oxygen atoms.
The alkane nitrites are considered an isomeric form of nitroalkane.
The alkyl nitrites are the esters of nitrous acid.
The nitro alkane can be classified into three classes depending upon the nature of carbon bonded to nitrogen atom:
Primary nitro alkane
When there is methane, or a normal alkyl chain present then it is called primary nitro alkane.

Image: general structure of primary nitro alkane.
Secondary nitro alkane
When the isopropyl group is attached to the nitro group it is called secondary nitro alkane.

Image: General structure of secondary nitro alkane.
Tertiary nitroalkane
When the tertiary carbon atom is fused with the nitro group then it is called tertiary nitroalkane.

Image: General structure of tertiary nitroalkane.
Hence, from the above discussion. Option (b) will be the correct answer because \[C{H_3}C{H_2}ONO\] does not contain direct nitrogen and carbon bonds.
Note: The organic compounds which contain the nitro group are acidic in nature. Nitro compounds are used in the manufacturing of detergents, dyes, pharmaceuticals, and explosives.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




