
Which of the following gives the silver mirror test?
A. Formic acid
B. \[C{H_3}COCHOHC{H_3}\]
C. Tartaric acid
D. Glucose
Answer
233.4k+ views
Hint: Silver mirror test is also termed as Tollens test. This test is used to differentiate between aldehydic group-containing compounds and ketonic group-containing compounds.
Complete Step by Step Solution:
Tollens reagent is a chemical reagent which helps in detecting the presence of an aldehyde functional group in the compound, alpha hydroxy ketone functional group in a compound and formic acid where its COOH group behaves like an aldehydic group.
The Tollens reagent is given by a German chemist known Bernhard Tollens. It is a solution mixture of silver nitrate and ammonia.
The compound gives a white precipitate of silver where silver salt reduces to silver metal and the aldehydic group get oxidises to silver salt of carboxylic acid.
A. Formic acid gives a positive silver mirror test as its COOH group act as an aldehydic group.
B. \[C{H_3}COCHOHC{H_3}\] has chemical name 3-hydroxybutan-2-one. It has an alpha hydroxy ketone functional group so it will give a positive silver mirror test.
C. Tartaric acid contains a COOH group which acts as an aldehydic group therefore it will give a silver mirror test.
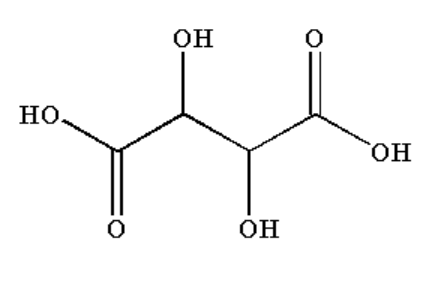
Image: Tartaric acid
D. Glucose contains alpha hydroxy ketone functional group so it will give a positive silver mirror test.
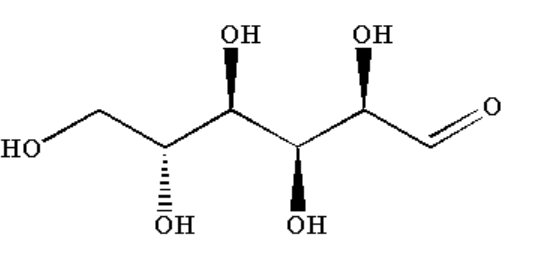
Image: Glucose
Thus, options A, B, C and D are correct.
Note: Ketone does not give a silver mirror test but alpha hydroxy ketone gives a silver mirror test. An alpha hydroxy ketone is a molecule having adjacent ketone and alcohol groups. Example: Glucose, tartaric acid.
Complete Step by Step Solution:
Tollens reagent is a chemical reagent which helps in detecting the presence of an aldehyde functional group in the compound, alpha hydroxy ketone functional group in a compound and formic acid where its COOH group behaves like an aldehydic group.
The Tollens reagent is given by a German chemist known Bernhard Tollens. It is a solution mixture of silver nitrate and ammonia.
The compound gives a white precipitate of silver where silver salt reduces to silver metal and the aldehydic group get oxidises to silver salt of carboxylic acid.
A. Formic acid gives a positive silver mirror test as its COOH group act as an aldehydic group.
B. \[C{H_3}COCHOHC{H_3}\] has chemical name 3-hydroxybutan-2-one. It has an alpha hydroxy ketone functional group so it will give a positive silver mirror test.
C. Tartaric acid contains a COOH group which acts as an aldehydic group therefore it will give a silver mirror test.

Image: Tartaric acid
D. Glucose contains alpha hydroxy ketone functional group so it will give a positive silver mirror test.

Image: Glucose
Thus, options A, B, C and D are correct.
Note: Ketone does not give a silver mirror test but alpha hydroxy ketone gives a silver mirror test. An alpha hydroxy ketone is a molecule having adjacent ketone and alcohol groups. Example: Glucose, tartaric acid.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




