
Which of the following gives benzoic acid on oxidation
A. Chlorophenol
B. Chlorotoluene
C. Chlorobenzene
D. Benzyl chloride
Answer
233.4k+ views
Hint: Oxidation is the addition of oxygen to a compound. It involves the loss of electrons increasing the oxidation state of a compound.
Complete Step by Step Solution:
Benzoic acid is an organic compound.
It is the simplest aromatic carboxylic acid.
It exists typically in many plants and is used as an intermediate in the biological synthesis of many secondary metabolites.
A. Chlorophenol
The chemical formula of chlorophenol is \[{{\rm{C}}_{\rm{6}}}{{\rm{H}}_{\rm{4}}}{\rm{OH}}\left( {{\rm{Cl}}} \right)\].
In this, there is a chlorine group attached to the ortho position of phenol.
The oxidation of this compound will give a combination of benzene diol compounds.
The reaction will occur as follows:
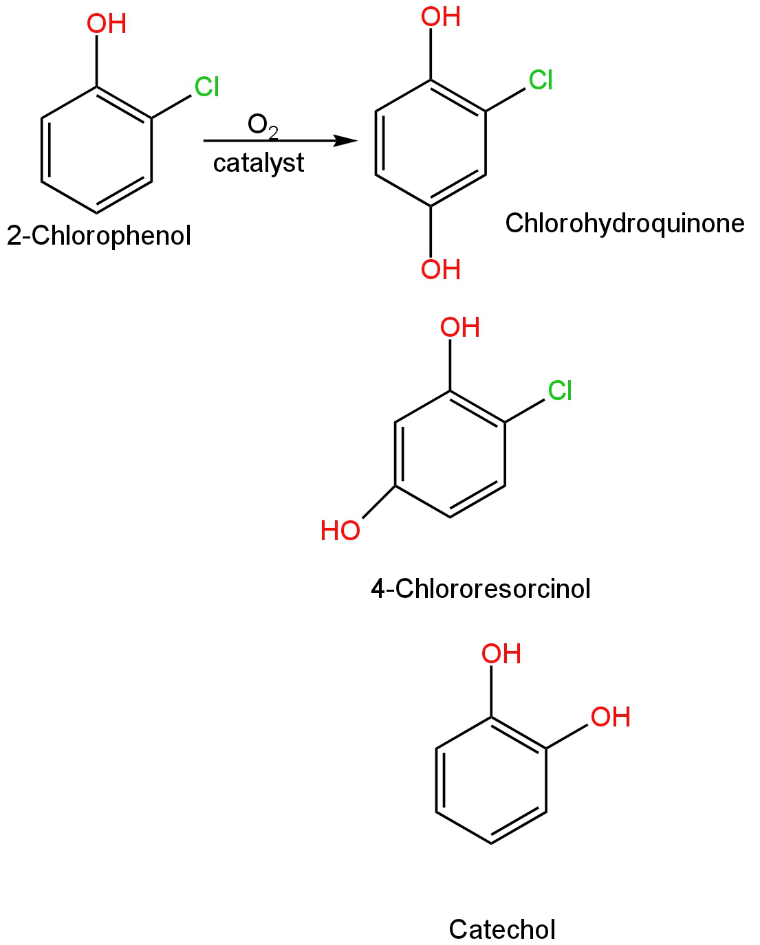
Image: Oxidation of chlorophenol.
It will not give benzoic acid as a product.
So, A is incorrect.
B. Chlorotoluene
The chemical formula of chlorotoluene is \[{{\rm{C}}_{\rm{6}}}{{\rm{H}}_{\rm{4}}}{\rm{C}}{{\rm{H}}_3}{\rm{(Cl)}}\].
In this, there is a chlorine group attached to the ortho position of toluene.
The oxidation of this compound will give chlorobenzoic acid.
The reaction will occur as follows:
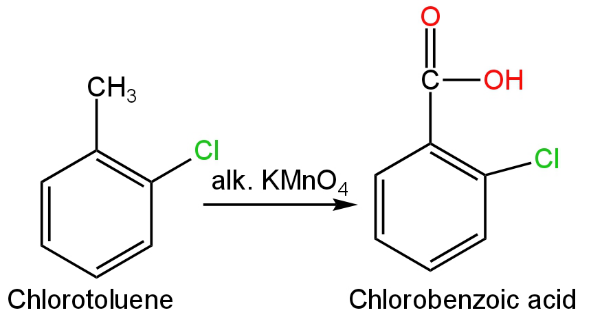
Image: Oxidation of chlorotoluene.
It will not give benzoic acid as a product.
So, B is incorrect.
C. Chlorobenzene
The chemical formula of chlorobenzene is \[{{\rm{C}}_{\rm{6}}}{{\rm{H}}_5}{\rm{Cl}}\].
In this, there is a chlorine group attached to the benzene.
The oxidation of this compound will not give benzoic acid.
So, C is incorrect.
D. Benzyl chloride
The chemical formula of benzyl chloride is \[{{\rm{C}}_{\rm{6}}}{{\rm{H}}_5}{\rm{C}}{{\rm{H}}_{\rm{2}}}{\rm{Cl}}\].
The oxidation of this compound will give benzoic acid.
The oxidation of this compound can be in the presence of alkaline potassium permanganate or potassium dichromate.
The reaction will occur as follows:
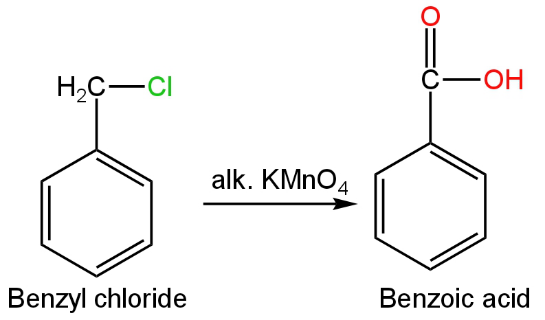
Image: Oxidation of benzyl chloride.
It will give benzoic acid as a product.
So, D is incorrect.
So, option D is correct.
Note: While attempting the question, one must know about the structures of every given option. The oxidation of benzyl chloride out of the given options will yield benzoic acid. Oxidation of chlorophenol, chlorotoluene, and chlorobenzene will yield benzene diols, chlorobenzoic acid, and ortho-substituted oxy-chlorobenzene respectively.
Complete Step by Step Solution:
Benzoic acid is an organic compound.
It is the simplest aromatic carboxylic acid.
It exists typically in many plants and is used as an intermediate in the biological synthesis of many secondary metabolites.
A. Chlorophenol
The chemical formula of chlorophenol is \[{{\rm{C}}_{\rm{6}}}{{\rm{H}}_{\rm{4}}}{\rm{OH}}\left( {{\rm{Cl}}} \right)\].
In this, there is a chlorine group attached to the ortho position of phenol.
The oxidation of this compound will give a combination of benzene diol compounds.
The reaction will occur as follows:

Image: Oxidation of chlorophenol.
It will not give benzoic acid as a product.
So, A is incorrect.
B. Chlorotoluene
The chemical formula of chlorotoluene is \[{{\rm{C}}_{\rm{6}}}{{\rm{H}}_{\rm{4}}}{\rm{C}}{{\rm{H}}_3}{\rm{(Cl)}}\].
In this, there is a chlorine group attached to the ortho position of toluene.
The oxidation of this compound will give chlorobenzoic acid.
The reaction will occur as follows:

Image: Oxidation of chlorotoluene.
It will not give benzoic acid as a product.
So, B is incorrect.
C. Chlorobenzene
The chemical formula of chlorobenzene is \[{{\rm{C}}_{\rm{6}}}{{\rm{H}}_5}{\rm{Cl}}\].
In this, there is a chlorine group attached to the benzene.
The oxidation of this compound will not give benzoic acid.
So, C is incorrect.
D. Benzyl chloride
The chemical formula of benzyl chloride is \[{{\rm{C}}_{\rm{6}}}{{\rm{H}}_5}{\rm{C}}{{\rm{H}}_{\rm{2}}}{\rm{Cl}}\].
The oxidation of this compound will give benzoic acid.
The oxidation of this compound can be in the presence of alkaline potassium permanganate or potassium dichromate.
The reaction will occur as follows:

Image: Oxidation of benzyl chloride.
It will give benzoic acid as a product.
So, D is incorrect.
So, option D is correct.
Note: While attempting the question, one must know about the structures of every given option. The oxidation of benzyl chloride out of the given options will yield benzoic acid. Oxidation of chlorophenol, chlorotoluene, and chlorobenzene will yield benzene diols, chlorobenzoic acid, and ortho-substituted oxy-chlorobenzene respectively.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




