
Which of the following derivatives of alcohols is unstable in an aqueous base?
A.
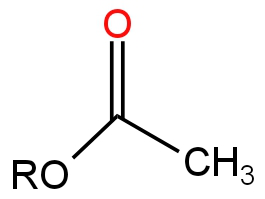
B.
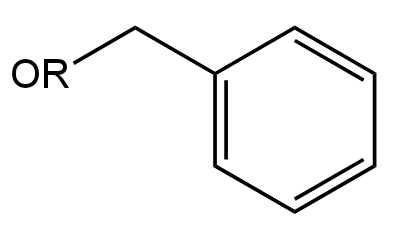
C.
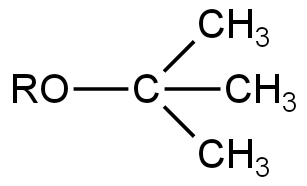
D.
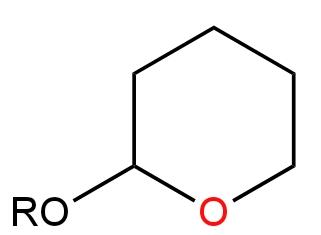
Answer
233.1k+ views
Hint: Base furnishes the Hydroxide ions which act as a nucleophile and attacks the Carbon atom which acts as an electrophile. The tendency of a compound to undergo a reaction in presence of a base depends on the electrophilic character of the Carbon atom attached to the Oxygen atom. If the Carbon atom has a more electrophilic character it will readily react with a base.
Complete Step by Step Solution:
A.
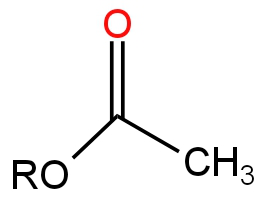
Image: Option A
It is an Ester.
Ester undergoes hydrolysis in the presence of a base by a nucleophilic substitution reaction.
This reaction happens through the Substitution nucleophilic bimolecular mechanism.
Step 1 - The hydroxide ion attacks the electrophilic carbon of the ester-forming the tetrahedral intermediate.
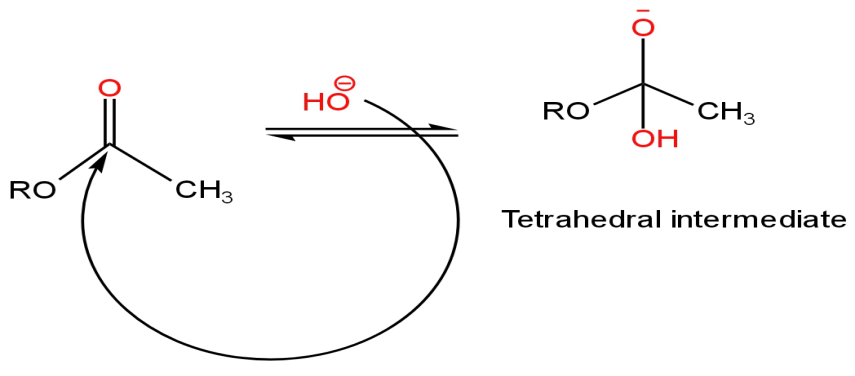
Image: Attack of the nucleophile on the carbonyl carbon
Step-2 - The intermediate breaks down forming Ethanoic acid with the loss of the leaving group (alkoxide).
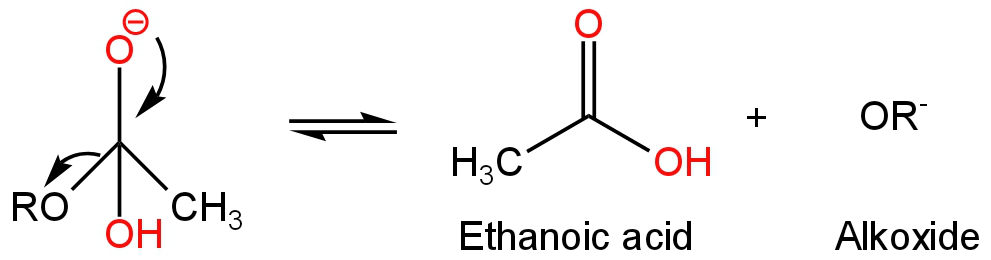
Image: Breaking down the intermediate
Step-3 - It is a fast reaction in which the alkoxide ions act as a base and deprotonate the Ethanoic acid-forming acetate ion and alcohol.
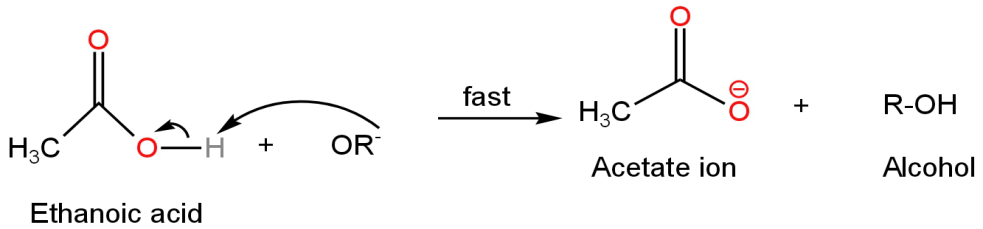
Image: Formation of ethanoic acid and alcohol
Thus, this compound is unstable in an aqueous base and hence will undergo hydrolysis.
B.
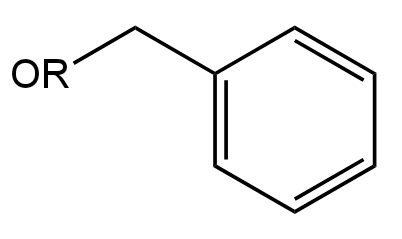
Image: Option B
This is an Ether compound.
Here the Carbon atom attached to the Oxygen atom is undergoing resonance with the phenyl group.
So, it will be difficult for the nucleophile to attack the carbon.
Thus, it will not undergo hydrolysis.
C.
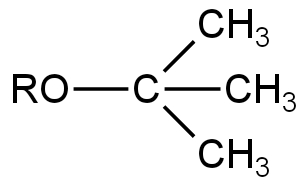
Image: Option C
This is also an Ether.
Here, due to the presence of three electron-donating methyl groups, the electrophilic character of the carbon atom attached to them is decreased.
So, it will not undergo any reaction in the presence of a base.
D.
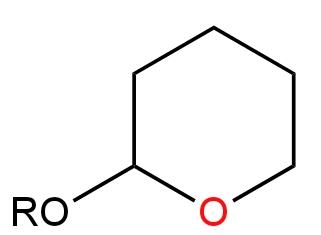
Image: Option D
There are two Ether groups here.
There is no carbon atom attached to the Oxygen atom. There is only a Methyl group.
Hence, it will not undergo any reaction in the presence of a base.
So, option A is correct.
Note: The base catalysis of an ester is a reaction irreversible as the end products are alcohol and carboxylate ion which is resonance stabilized and thus stable and does not react with alcohol.
Complete Step by Step Solution:
A.

Image: Option A
It is an Ester.
Ester undergoes hydrolysis in the presence of a base by a nucleophilic substitution reaction.
This reaction happens through the Substitution nucleophilic bimolecular mechanism.
Step 1 - The hydroxide ion attacks the electrophilic carbon of the ester-forming the tetrahedral intermediate.

Image: Attack of the nucleophile on the carbonyl carbon
Step-2 - The intermediate breaks down forming Ethanoic acid with the loss of the leaving group (alkoxide).

Image: Breaking down the intermediate
Step-3 - It is a fast reaction in which the alkoxide ions act as a base and deprotonate the Ethanoic acid-forming acetate ion and alcohol.

Image: Formation of ethanoic acid and alcohol
Thus, this compound is unstable in an aqueous base and hence will undergo hydrolysis.
B.

Image: Option B
This is an Ether compound.
Here the Carbon atom attached to the Oxygen atom is undergoing resonance with the phenyl group.
So, it will be difficult for the nucleophile to attack the carbon.
Thus, it will not undergo hydrolysis.
C.

Image: Option C
This is also an Ether.
Here, due to the presence of three electron-donating methyl groups, the electrophilic character of the carbon atom attached to them is decreased.
So, it will not undergo any reaction in the presence of a base.
D.

Image: Option D
There are two Ether groups here.
There is no carbon atom attached to the Oxygen atom. There is only a Methyl group.
Hence, it will not undergo any reaction in the presence of a base.
So, option A is correct.
Note: The base catalysis of an ester is a reaction irreversible as the end products are alcohol and carboxylate ion which is resonance stabilized and thus stable and does not react with alcohol.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




