
Which of the following arrangements of molecules is correct on the basis of their dipole moments?
(A) $B{F_3} > N{F_3} > N{H_3}$
(B) $N{F_3} > B{F_3} > N{H_3}$
(C) $N{H_3} > B{F_3} > N{F_3}$
(D) $N{H_3} > N{F_3} > B{F_3}$
Answer
233.1k+ views
Hint: How far away two electrical charges are from one another is measured in terms of a dipole moment. Dipole moments are a type of vector. The direction is from negative charge to positive charge, and the magnitude is equal to the charge times the distance between the charges. The formula of the dipole moment is $p = qd$ .
Complete step by step solution:
As we discussed above some characteristics of Dipole moment, let we consider the correct arrangement for the molecules on the basis of their dipole moment,
We know that, In $N{H_3}$ , the charge from three bonds was delocalised in the direction of lone pairs, towards nitrogen.
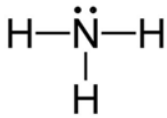
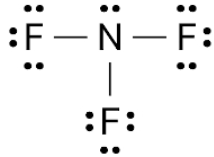
$N{F_3}$ has a greater dipole moment than $B{F_3}$ as a result of its pyramidal and trigonal planar structures. For $B{F_3}$ , there is no resulting dipole.
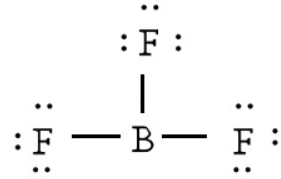
Therefore, from the above conclusion we get the correct sequence is $N{H_3} > N{F_3} > B{F_3}$ .
Hence, the correct option is (D).
Note: The dipole moment is helpful in determining how polar the chemical connection is: The bond becomes more polar as the size of the dipole moment grows. Non-polar molecules are those that have no dipole moment.
Complete step by step solution:
As we discussed above some characteristics of Dipole moment, let we consider the correct arrangement for the molecules on the basis of their dipole moment,
We know that, In $N{H_3}$ , the charge from three bonds was delocalised in the direction of lone pairs, towards nitrogen.
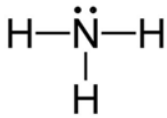

$N{F_3}$ has a greater dipole moment than $B{F_3}$ as a result of its pyramidal and trigonal planar structures. For $B{F_3}$ , there is no resulting dipole.
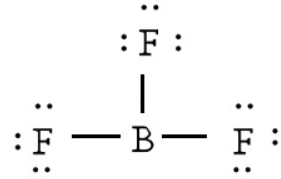
Therefore, from the above conclusion we get the correct sequence is $N{H_3} > N{F_3} > B{F_3}$ .
Hence, the correct option is (D).
Note: The dipole moment is helpful in determining how polar the chemical connection is: The bond becomes more polar as the size of the dipole moment grows. Non-polar molecules are those that have no dipole moment.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




