
Which of the following are formed on the addition reaction of DCI with 3-methyl-1-butene
A. \[C{H_2}DCHClCH{\left( {C{H_3}} \right)_2}\]
B. \[C{H_2}DC{H_2}CCl{\left( {C{H_3}} \right)_2}\]
C. \[C{H_3}CDClCH{\left( {C{H_3}} \right)_2}\]
D.\[ClC{H_2}CHDCH{\left( {C{H_3}} \right)_2}\]
Answer
233.1k+ views
Hint: DCl is deuterium chloride. Deuterium is one of two stable isotopes of hydrogen.
The addition of DCl to 3-methyl-1-butene occurs through an electrophilic addition reaction.
Complete Step by Step Answer:
DCl is deuterium chloride which is one of two stable isotopes of hydrogen. The nucleus of a deuterium atom contains one proton and one neutron. An electrophilic addition reaction is a reaction where the substrate is first attacked by an electrophile leading to the addition of one or more molecules across multiple bonds.
The double bond in alkenes is electron affluent due to the existence of 4 electrons rather than two in a single bond. So, the double bonds can effortlessly provide lone pair electrons to act like a nucleophile. A nucleophile is a nucleus-loving, electron-rich Lewis acid.
During electrophilic addition reactions, double bonds provide lone pair electrons to an electrophile. It is an electron-loving, electron-poor Lewis base. The pi bond of the alkene is broken down to establish two individual sigma bonds.
The reaction happens according to Markovnikov's rule which states that with the addition of a protic acid or other polar reagents to an asymmetrical alkene, the electropositive portion gets connected to the carbon with additional hydrogen substituents, and the electronegative portion gets connected to the carbon with more alkyl substituents.
Mechanism
Step-1
DCl dissociates to form \[{D^ + }\] and\[C{l^ - }\].
The electrophile D+ establishes a covalent bond with an electron-rich\[C = C\] bond.
The positive charge on Cl is shifted to the carbon-carbon bond, constructing a carbocation during the formation of the C-D bond.
A secondary carbocation is more stable than a primary carbocation.
Step-2
The positively charged intermediate combines with an electron-rich species Cl- to form the second covalent bond.
The mechanism happens as follows:
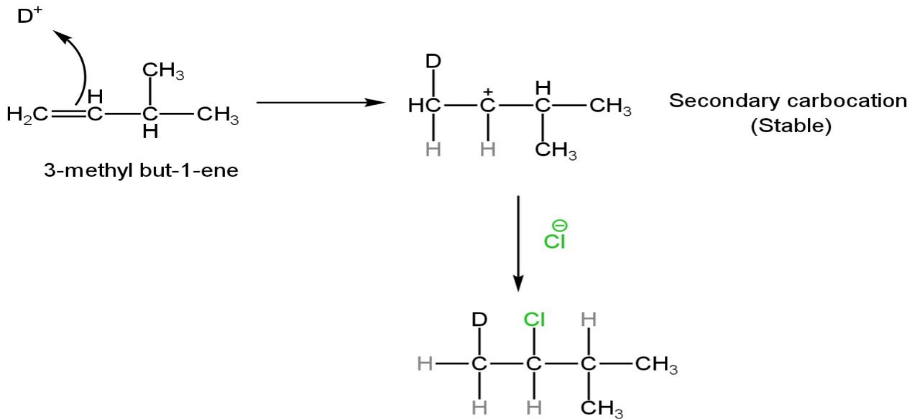
Image: Electrophilic addition reaction of DCI with 3-methyl-1-butene.
So, the structure of the product formed is \[C{H_2}DCHClCH{\left( {C{H_3}} \right)_2}\].
So, option A is correct.
Note: In organic chemistry, Markovnikov's rule interprets the result of some addition reactions. The rule was developed by Russian chemist Vladimir Markovnikov in 1870. Anti Markownikoff rule or Kharasch effect involves the reactions that do not comprise a carbocation intermediate and may react through other mechanisms in which Markovnikov's rule cannot be applied, like free radical addition.
The addition of DCl to 3-methyl-1-butene occurs through an electrophilic addition reaction.
Complete Step by Step Answer:
DCl is deuterium chloride which is one of two stable isotopes of hydrogen. The nucleus of a deuterium atom contains one proton and one neutron. An electrophilic addition reaction is a reaction where the substrate is first attacked by an electrophile leading to the addition of one or more molecules across multiple bonds.
The double bond in alkenes is electron affluent due to the existence of 4 electrons rather than two in a single bond. So, the double bonds can effortlessly provide lone pair electrons to act like a nucleophile. A nucleophile is a nucleus-loving, electron-rich Lewis acid.
During electrophilic addition reactions, double bonds provide lone pair electrons to an electrophile. It is an electron-loving, electron-poor Lewis base. The pi bond of the alkene is broken down to establish two individual sigma bonds.
The reaction happens according to Markovnikov's rule which states that with the addition of a protic acid or other polar reagents to an asymmetrical alkene, the electropositive portion gets connected to the carbon with additional hydrogen substituents, and the electronegative portion gets connected to the carbon with more alkyl substituents.
Mechanism
Step-1
DCl dissociates to form \[{D^ + }\] and\[C{l^ - }\].
The electrophile D+ establishes a covalent bond with an electron-rich\[C = C\] bond.
The positive charge on Cl is shifted to the carbon-carbon bond, constructing a carbocation during the formation of the C-D bond.
A secondary carbocation is more stable than a primary carbocation.
Step-2
The positively charged intermediate combines with an electron-rich species Cl- to form the second covalent bond.
The mechanism happens as follows:

Image: Electrophilic addition reaction of DCI with 3-methyl-1-butene.
So, the structure of the product formed is \[C{H_2}DCHClCH{\left( {C{H_3}} \right)_2}\].
So, option A is correct.
Note: In organic chemistry, Markovnikov's rule interprets the result of some addition reactions. The rule was developed by Russian chemist Vladimir Markovnikov in 1870. Anti Markownikoff rule or Kharasch effect involves the reactions that do not comprise a carbocation intermediate and may react through other mechanisms in which Markovnikov's rule cannot be applied, like free radical addition.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




