
Water is liquid due to:
(a) Hydrogen bonding
(b) Covalent bonding
(c) Ionic bonding
(d) Vander Waals force
Answer
232.8k+ views
Hint: Water is a molecule consisting of oxygen and hydrogen. Water is represented by the formula of H2O. The existence of water in three states of matter namely water (liquid), ice (solid) and steam (gas) are important.
Complete Step by Step Solution:
Here, we have found out the reason for the liquid state of water. Let's discuss all the options one by one.
Let's understand the covalent bond. The formation of a covalent bond takes place due to the electron shared between the atoms involved in the formation of bond. But, the water is not liquid due to the covalent bond.
Now, we will understand the ionic bond. This bond forms due to the attraction of ions which possess opposite charges. The formation of the molecule NaCl is an example of ionic bond.
Van der Waals force defines the force that is the intermolecular force which exists for a short time between all types of molecules, ions and atoms when they come close to each other in sufficient manner. But this is not the reason for the liquid state of water.
Now, we will discuss the hydrogen bond in detail. The formation of a hydrogen bond takes place when the bonding of a hydrogen atom happens with an electronegative atom like N, O, F.
Let's discuss hydrogen bonding in water. Here, the attraction of a H atom of one molecule of water happens with the O atom of the neighbouring water molecule.
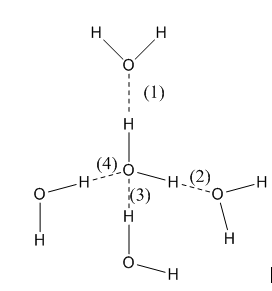
Image: Hydrogen bonding in water
So, we see that four molecules of water are bonded to a water molecule through hydrogen bonding. This causes enough force to hold the molecules of water together. Therefore, water exists in the liquid state.
Hence, option (a) is right.
Note: The hydrogen bond's nature is weaker to an ionic bond. Its strength is between the covalent bond which is strong in nature and Van der Waals force which is weak. It is actually the dipole-dipole interaction of molecules.
Complete Step by Step Solution:
Here, we have found out the reason for the liquid state of water. Let's discuss all the options one by one.
Let's understand the covalent bond. The formation of a covalent bond takes place due to the electron shared between the atoms involved in the formation of bond. But, the water is not liquid due to the covalent bond.
Now, we will understand the ionic bond. This bond forms due to the attraction of ions which possess opposite charges. The formation of the molecule NaCl is an example of ionic bond.
Van der Waals force defines the force that is the intermolecular force which exists for a short time between all types of molecules, ions and atoms when they come close to each other in sufficient manner. But this is not the reason for the liquid state of water.
Now, we will discuss the hydrogen bond in detail. The formation of a hydrogen bond takes place when the bonding of a hydrogen atom happens with an electronegative atom like N, O, F.
Let's discuss hydrogen bonding in water. Here, the attraction of a H atom of one molecule of water happens with the O atom of the neighbouring water molecule.

Image: Hydrogen bonding in water
So, we see that four molecules of water are bonded to a water molecule through hydrogen bonding. This causes enough force to hold the molecules of water together. Therefore, water exists in the liquid state.
Hence, option (a) is right.
Note: The hydrogen bond's nature is weaker to an ionic bond. Its strength is between the covalent bond which is strong in nature and Van der Waals force which is weak. It is actually the dipole-dipole interaction of molecules.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




