
The value of bond order in $N{O^ + }$ according to molecular orbital theory is:
(A) 3
(B) 2
(C) 1
(D) 0
Answer
233.1k+ views
Hint: Bond order is the difference between the number of bonding and anti bonding electrons, divided by two. So first find out the total number of electrons present in $N{O^ + }$ ion and write down its configuration. From this find out the number of bonding and anti bonding electrons and use them in the formula.
Formula used:
Bond order: BO = $\dfrac{1}{2}$ (Bonding electrons – Anti bonding electrons) (1)
Complete step by step answer:
- First we will talk about the Molecular orbital theory.
In the molecular orbital theory the electronic configuration or structure of the molecules by the use of quantum mechanics. Atomic orbitals combine and form molecular orbitals. According to this theory:
(1)The total number of atomic orbitals will be equal to the total number of molecular orbitals.
(2)The molecular orbitals are of 3 types: bonding molecular orbitals, non-bonding molecular orbitals and anti-bonding molecular orbitals. The energy of the anti-bonding molecular orbitals is more than the energy of the parent orbitals and the energy of the bonding molecular orbitals is always lower than the parent orbitals.
(3)The electrons are filled in the molecular orbitals according to the increasing order of energy.
(4)When the combining atomic orbitals have similar energies, the molecular orbitals formed are most effective.
-Now let us see what bond order is.
Bond order is basically the difference between the number of bonding and anti bonding electrons, divided by two. Mathematically it can be written as:
BO = $\dfrac{1}{2}$ (Bonding electrons – Anti bonding electrons) (1)
Now we will check the number of atoms present in $N{O^ + }$.
The number of electrons in this molecules will be = 7 + 8 – 1 = 14 electrons
Hence its electronic configuration will be: $\sigma 1{s^2}, \sigma *1{s^2}, \sigma 2{s^2}, \sigma *2{s^2}, \pi 2p{x^2}, \pi 2p{y^2}, \sigma 2p{z^2}$
Its molecular orbital diagram will be as follows:
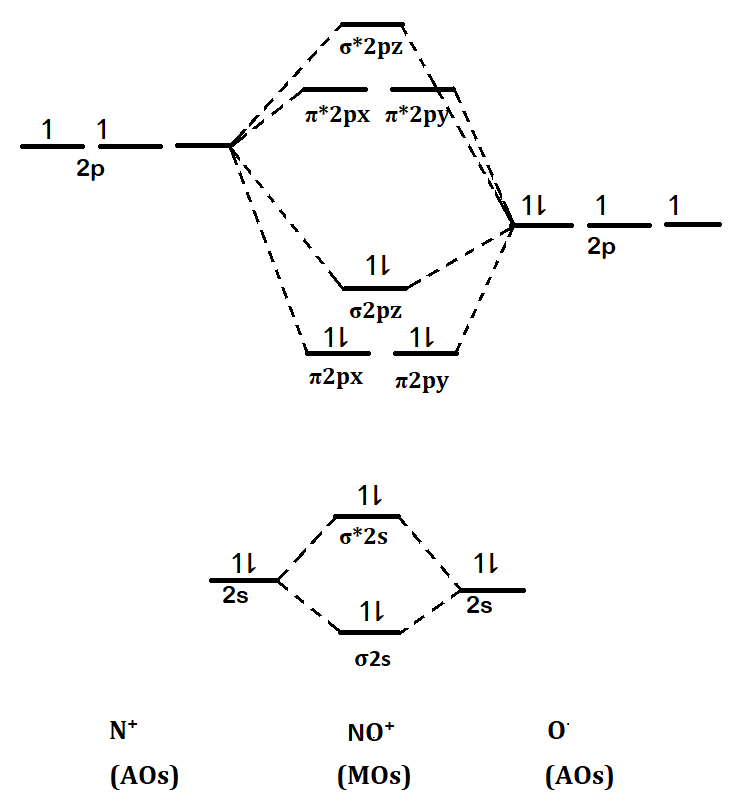
Here we can see that the number of bonding electrons are = 8; and the number of anti bonding electrons are = 2.
Now using equation (1) we will find out its bond order:
BO = $\dfrac{1}{2}$ (Bonding electrons – Anti bonding electrons)
= $\dfrac{1}{2}$ (8 – 2)
= 3
Hence the correct option will be: (A) 3
Note: The MOT explains the existence of a molecule on the basis of bond order, but this concept is neither feasible nor appropriate to explain molecular existence of polyatomic molecules. Also MOT does not say anything about the geometry and shape of the molecule. So, this theory also has some drawbacks.
Formula used:
Bond order: BO = $\dfrac{1}{2}$ (Bonding electrons – Anti bonding electrons) (1)
Complete step by step answer:
- First we will talk about the Molecular orbital theory.
In the molecular orbital theory the electronic configuration or structure of the molecules by the use of quantum mechanics. Atomic orbitals combine and form molecular orbitals. According to this theory:
(1)The total number of atomic orbitals will be equal to the total number of molecular orbitals.
(2)The molecular orbitals are of 3 types: bonding molecular orbitals, non-bonding molecular orbitals and anti-bonding molecular orbitals. The energy of the anti-bonding molecular orbitals is more than the energy of the parent orbitals and the energy of the bonding molecular orbitals is always lower than the parent orbitals.
(3)The electrons are filled in the molecular orbitals according to the increasing order of energy.
(4)When the combining atomic orbitals have similar energies, the molecular orbitals formed are most effective.
-Now let us see what bond order is.
Bond order is basically the difference between the number of bonding and anti bonding electrons, divided by two. Mathematically it can be written as:
BO = $\dfrac{1}{2}$ (Bonding electrons – Anti bonding electrons) (1)
Now we will check the number of atoms present in $N{O^ + }$.
The number of electrons in this molecules will be = 7 + 8 – 1 = 14 electrons
Hence its electronic configuration will be: $\sigma 1{s^2}, \sigma *1{s^2}, \sigma 2{s^2}, \sigma *2{s^2}, \pi 2p{x^2}, \pi 2p{y^2}, \sigma 2p{z^2}$
Its molecular orbital diagram will be as follows:

Here we can see that the number of bonding electrons are = 8; and the number of anti bonding electrons are = 2.
Now using equation (1) we will find out its bond order:
BO = $\dfrac{1}{2}$ (Bonding electrons – Anti bonding electrons)
= $\dfrac{1}{2}$ (8 – 2)
= 3
Hence the correct option will be: (A) 3
Note: The MOT explains the existence of a molecule on the basis of bond order, but this concept is neither feasible nor appropriate to explain molecular existence of polyatomic molecules. Also MOT does not say anything about the geometry and shape of the molecule. So, this theory also has some drawbacks.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




