
The structure of naturally occurring steroid cholesterol is given:
Which of the following statements are correct?
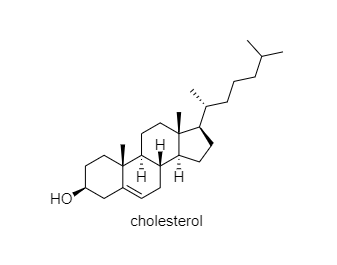
This question has multiple correct options.
(A) There are nine chiral centers in the cholesterol
(B) It is a pentacyclic compound
(C) There are two quaternary carbon atoms in the compound
(D) There are six tertiary carbon atoms in the compound
Answer
233.1k+ views
Hint: Cholesterol: It is a sterol (or modified steroid), a lipid molecule, and is biosynthesized by all animal cells because it is an essential structural component of all animals (not plant or bacterial) cell membranes that are required to maintain both membrane structural integrity and fluidity.
Complete step by step solution:
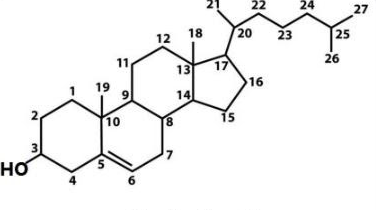
> A chiral center is defined as an atom in a molecule that is bonded to four different chemical species, allowing for optical isomerism. There are eight chiral centers in cholesterol. There is no internal plane of symmetry so each carbon is different.
> Cholesterol contains four rings. So, it is a tetracyclic compound.
> Quaternary carbon atom: It is a carbon atom bonded to the other four carbon atoms.
> In cholesterol, there are four alicyclic rings and two quaternary carbon atoms in the compound which are ${ C-10 }$ and ${ C-13 }$.
> There are six tertiary carbon atoms in the compound which are ${ C-8 }, { C-9 }, { C-14 }, { C-17 }, { C-20 }, and { C-25 }$.
Hence, the correct option is B.
Note:The possibility to make a mistake is that you may choose option C as there are four quaternary carbons but there are four alicyclic rings and two quaternary carbon atoms.
Complete step by step solution:

> A chiral center is defined as an atom in a molecule that is bonded to four different chemical species, allowing for optical isomerism. There are eight chiral centers in cholesterol. There is no internal plane of symmetry so each carbon is different.
> Cholesterol contains four rings. So, it is a tetracyclic compound.
> Quaternary carbon atom: It is a carbon atom bonded to the other four carbon atoms.
> In cholesterol, there are four alicyclic rings and two quaternary carbon atoms in the compound which are ${ C-10 }$ and ${ C-13 }$.
> There are six tertiary carbon atoms in the compound which are ${ C-8 }, { C-9 }, { C-14 }, { C-17 }, { C-20 }, and { C-25 }$.
Hence, the correct option is B.
Note:The possibility to make a mistake is that you may choose option C as there are four quaternary carbons but there are four alicyclic rings and two quaternary carbon atoms.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




