
The rate at which a substance reacts depends on its –
A. Atomic mass
B. Equivalent mass
C. Molecular mass
D. Molar concentration
Answer
232.8k+ views
Hint: We know that molar concentration is the amount of solute present per unit volume of solution. Rate of reaction is the speed with which reaction proceeds to completion.
Complete step by step solution:
Rate of reaction is the speed at which reactants are converted into products. Some chemical reactions occur instantaneously while some take a great amount of time. There are many factors which affect the rate of reaction. Concentration of the reactants is the major factor in this.
Rate law provides a relation between concentrations of reactants and rate of reaction.
Let us consider the reaction \[{\text{aA + bB }} \to {\text{ cC + dD}}\]
Where, a and b are the stoichiometric coefficients of the reactant and c and d are that of products. Rate law for this reaction is given as - \[{\text{Rate }} \propto {\text{ [A}}{{\text{]}}^{\text{x}}}{\text{.[B}}{{\text{]}}^{\text{y}}}\]
i.e. \[{\text{Rate = k}}{\text{.[A}}{{\text{]}}^{\text{x}}}{\text{.[B}}{{\text{]}}^{\text{y}}}\]
where, k is the rate constant of the reaction and x and y are the partial reaction orders for the reactants A and B.
As we can see the rate of the reaction is directly proportional to the concentration of reactant raised to the power of their stoichiometric coefficients.
Thus, we can infer that as the concentration of reactant increases, rate of reaction also increases and as concentration of reactant decreases the rate of reaction also decreases. Time plays a major role here.
For much better understanding let’s take a look at this graph –
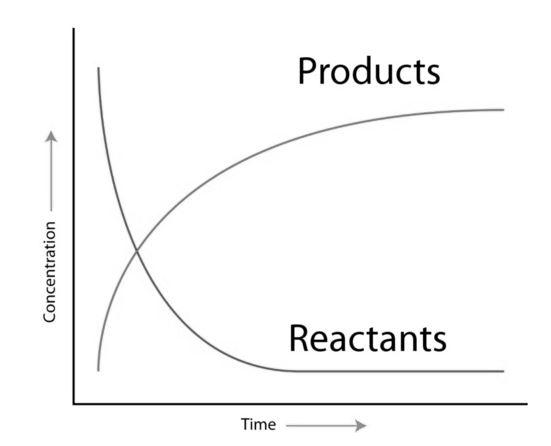
When the reaction begins, the concentration of reactants is high i.e. a large number of reactant molecules are available for conversion to product. So, the reaction begins faster. But as the reaction proceeds, concentration of the reactant goes on decreasing as more and more of it gets converted to product. So, the concentration of product increases. Eventually as there is less and less reactant available for conversion, the reaction slows down. At a point, there is no reactant left to convert to product. So, the reaction will stop.
So, we can infer that rate at which a substance reacts depends on its molar concentration.
Hence, option D is correct.
Note: Remember that mass and concentration of a substance are two different concepts. Speed at which a reaction will proceed will depend more on the concentration.
Complete step by step solution:
Rate of reaction is the speed at which reactants are converted into products. Some chemical reactions occur instantaneously while some take a great amount of time. There are many factors which affect the rate of reaction. Concentration of the reactants is the major factor in this.
Rate law provides a relation between concentrations of reactants and rate of reaction.
Let us consider the reaction \[{\text{aA + bB }} \to {\text{ cC + dD}}\]
Where, a and b are the stoichiometric coefficients of the reactant and c and d are that of products. Rate law for this reaction is given as - \[{\text{Rate }} \propto {\text{ [A}}{{\text{]}}^{\text{x}}}{\text{.[B}}{{\text{]}}^{\text{y}}}\]
i.e. \[{\text{Rate = k}}{\text{.[A}}{{\text{]}}^{\text{x}}}{\text{.[B}}{{\text{]}}^{\text{y}}}\]
where, k is the rate constant of the reaction and x and y are the partial reaction orders for the reactants A and B.
As we can see the rate of the reaction is directly proportional to the concentration of reactant raised to the power of their stoichiometric coefficients.
Thus, we can infer that as the concentration of reactant increases, rate of reaction also increases and as concentration of reactant decreases the rate of reaction also decreases. Time plays a major role here.
For much better understanding let’s take a look at this graph –

When the reaction begins, the concentration of reactants is high i.e. a large number of reactant molecules are available for conversion to product. So, the reaction begins faster. But as the reaction proceeds, concentration of the reactant goes on decreasing as more and more of it gets converted to product. So, the concentration of product increases. Eventually as there is less and less reactant available for conversion, the reaction slows down. At a point, there is no reactant left to convert to product. So, the reaction will stop.
So, we can infer that rate at which a substance reacts depends on its molar concentration.
Hence, option D is correct.
Note: Remember that mass and concentration of a substance are two different concepts. Speed at which a reaction will proceed will depend more on the concentration.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




