
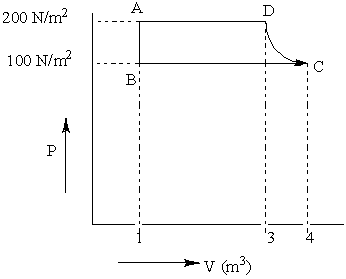
The P-V diagram of a diatomic ideal gas going under cyclic process as shown in the figure. The work done during an adiabatic process CD is (use \[\gamma \] =1.4):
(1) - 500 J
(2) - 400 J
(3) 400 J
(4) 200 J
Answer
233.1k+ views
Hint: An adiabatic process defines a process in thermodynamics where transferring of energy takes place without the transfer of mass or heat to the surrounding.
Formula used:
The formula for the work done is,
\[W = \dfrac{{{p_f}{v_f} - {p_i}{v_i}}}{{1 - \gamma }}\]
Here, W is work done, \[{p_f}\] is the final pressure, \[{p_i}\] is the initial pressure, \[{v_f}\] is the final volume and \[{v_i}\] is the initial volume.
Complete Step by Step Solution:
The Given diagram represents the adiabatic process of an ideal gas. From the graph, \[{p_i}\]is 100 N/m2 and \[{p_f}\]is 200 N/m2 . And \[{v_f}\]is 3 and \[{v_i}\]is 4 and \[\gamma \]is 1.4.
Putting all the above values in the formula of W.
So, work done in adiabatic process is,
\[\] \[{W_{CD}} = \dfrac{{200 \times 3 - 4 \times 100}}{{1 - 1.4}}\]
\[{W_{CD}} = \dfrac{{600 - 400}}{{ - 0.4}}\]
\[{W_{CD}} = - 500\,J\]
Hence, the work done in the given adiabatic process is -500 J. Therefore, option (1) is right
Additional Information:
Thermodynamics defines the studying of relations between work, energy, heat and temperature. The different thermodynamic law explains how the change of energy of a system takes place and whether the system has the ability to perform important work on the surroundings.
Note: There is the possibility of both reversibility or irreversibility of an adiabatic process. The necessary conditions required for the adiabatic process: (a)Perfect insulation of the system from the surrounding (b) The process should happen quickly so that there is enough time for the taking place of heat transfer.
Formula used:
The formula for the work done is,
\[W = \dfrac{{{p_f}{v_f} - {p_i}{v_i}}}{{1 - \gamma }}\]
Here, W is work done, \[{p_f}\] is the final pressure, \[{p_i}\] is the initial pressure, \[{v_f}\] is the final volume and \[{v_i}\] is the initial volume.
Complete Step by Step Solution:
The Given diagram represents the adiabatic process of an ideal gas. From the graph, \[{p_i}\]is 100 N/m2 and \[{p_f}\]is 200 N/m2 . And \[{v_f}\]is 3 and \[{v_i}\]is 4 and \[\gamma \]is 1.4.
Putting all the above values in the formula of W.
So, work done in adiabatic process is,
\[\] \[{W_{CD}} = \dfrac{{200 \times 3 - 4 \times 100}}{{1 - 1.4}}\]
\[{W_{CD}} = \dfrac{{600 - 400}}{{ - 0.4}}\]
\[{W_{CD}} = - 500\,J\]
Hence, the work done in the given adiabatic process is -500 J. Therefore, option (1) is right
Additional Information:
Thermodynamics defines the studying of relations between work, energy, heat and temperature. The different thermodynamic law explains how the change of energy of a system takes place and whether the system has the ability to perform important work on the surroundings.
Note: There is the possibility of both reversibility or irreversibility of an adiabatic process. The necessary conditions required for the adiabatic process: (a)Perfect insulation of the system from the surrounding (b) The process should happen quickly so that there is enough time for the taking place of heat transfer.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




