
The product of acid-catalysed hydration of $2-phenylpropene$ is
A. $3-phenyl-2-propanol$
B. $1-phenyl-2-propanol$
C. $2-phenyl-2-propanol$
D. $2-phenyl-1-propanol$
Answer
233.1k+ views
Hint: Acid-catalysed hydration is the addition of water to an alkene that forms alcohol. This is one kind of electrophilic hydration and here the $-OH$ group is attached across the two carbon atoms of a double bond. Here we have a substituted alkene undergoing acid-catalysed hydration to give an alcohol.
Complete Step by Step Answer:
As we know alcohol gets dehydrated to form an alkene, in the same way, alkenes are hydrated to form alcohol. Acid-catalysed hydration of alkenes produces alcohol according to Markownikov’s rule. This process involves the breaking of the pi bond in the alkene and hydroxyl bond ($-OH$) in the water molecule.
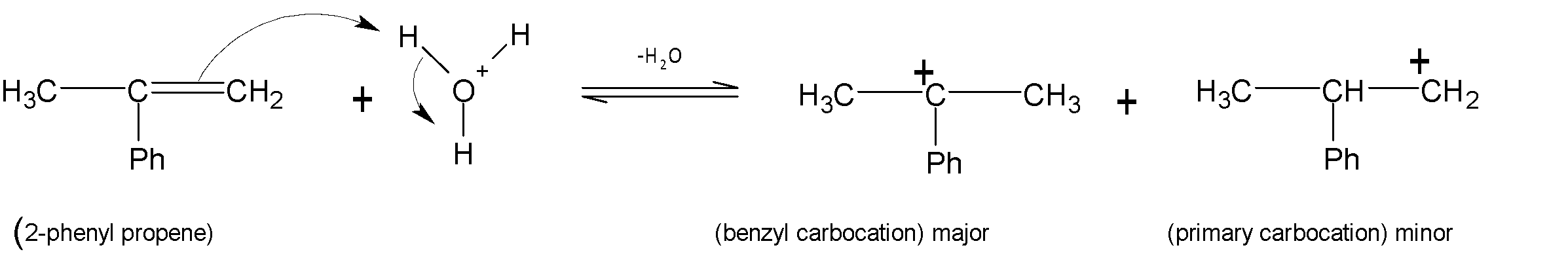
$2-phenyl$propene undergoes acid-catalysed hydration to give alcohol by the following step. The first step involves the protonation of alkenes to form the most stable carbocation by the electrophilic attack ${{H}_{3}}{{O}^{+}}$. Here two carbocations are formed but benzylic carbocations are more stable. This is because conjugation with phenyl rings gives them stability.
In the second step, water acts as an incoming nucleophile and hence attacks carbocation.
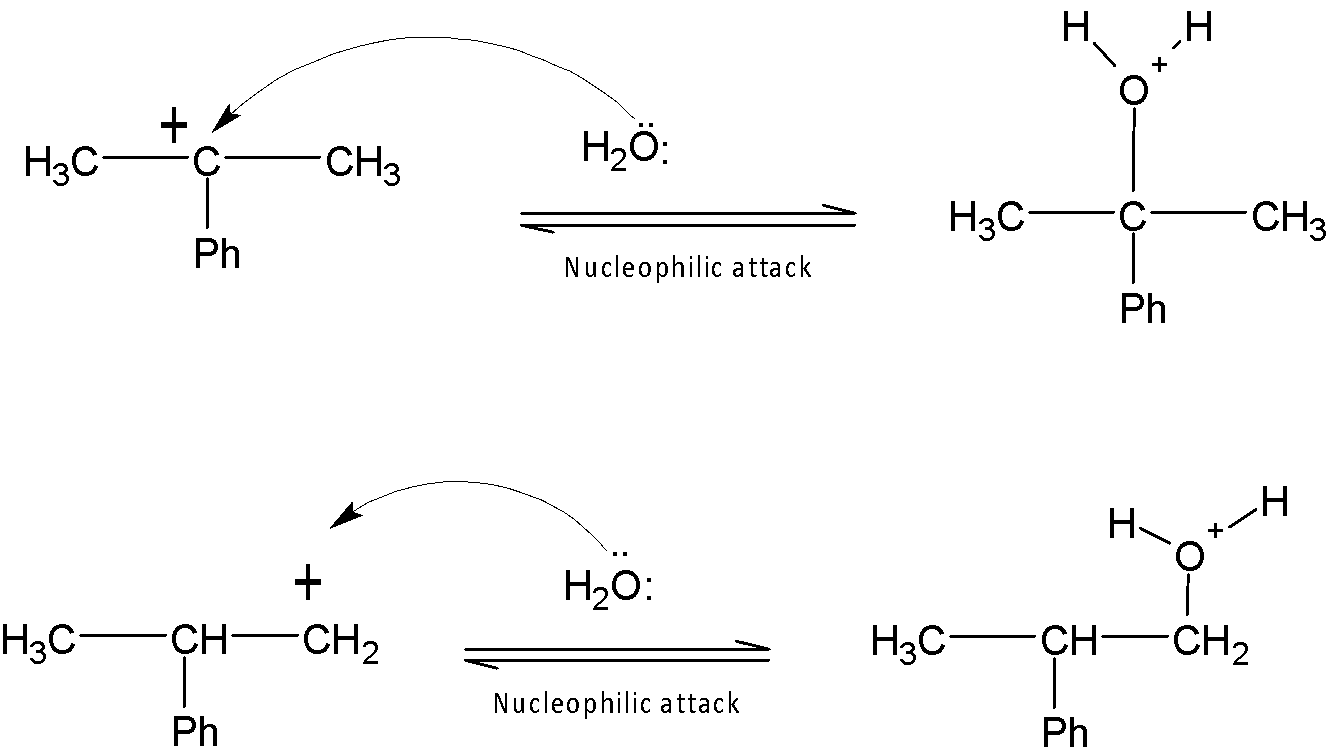
The final and last step is the deprotonation step. Alcohol named as $2-phenyl-2-propanol$is formed as the major product by deprotonation and $2-phenyl-1-propanol$is the minor product.

Therefore, the product of acid-catalysed hydration $2-phenyl-propanol$.
Thus, option (C) is correct.
Note: The acid-catalysed hydration of alkene is regioselective in nature and also follows Markovnikov’s rule. The regioselectivity of acid-catalysed hydration shows that the hydroxyl group ($-OH$) gets attached to the more substituted carbon in an alkene.
Complete Step by Step Answer:
As we know alcohol gets dehydrated to form an alkene, in the same way, alkenes are hydrated to form alcohol. Acid-catalysed hydration of alkenes produces alcohol according to Markownikov’s rule. This process involves the breaking of the pi bond in the alkene and hydroxyl bond ($-OH$) in the water molecule.
$2-phenyl$propene undergoes acid-catalysed hydration to give alcohol by the following step. The first step involves the protonation of alkenes to form the most stable carbocation by the electrophilic attack ${{H}_{3}}{{O}^{+}}$. Here two carbocations are formed but benzylic carbocations are more stable. This is because conjugation with phenyl rings gives them stability.

In the second step, water acts as an incoming nucleophile and hence attacks carbocation.

The final and last step is the deprotonation step. Alcohol named as $2-phenyl-2-propanol$is formed as the major product by deprotonation and $2-phenyl-1-propanol$is the minor product.

Therefore, the product of acid-catalysed hydration $2-phenyl-propanol$.
Thus, option (C) is correct.
Note: The acid-catalysed hydration of alkene is regioselective in nature and also follows Markovnikov’s rule. The regioselectivity of acid-catalysed hydration shows that the hydroxyl group ($-OH$) gets attached to the more substituted carbon in an alkene.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




