
The nature of hybridization in the \[BC{l_3}\]molecule is
(a) \[sp\]
(b) \[s{p^2}\]
(c) \[s{p^3}\]
(d) \[s{p^3}d\]
Answer
233.4k+ views
Hint: With the help of the concept of hybridization, we can easily predict the structure of any given molecule along with the bond angle. The \[s{p^3}\], \[s{p^2}\]and \[sp\]hybridization represents the tetrahedral, planar, and linear geometries respectively.
Complete step by step solution:The \[BC{l_3}\]molecule is known as boron trichloride.
In the \[BC{l_3}\]molecule, the boron atom occupies a central position and is connected with three chlorine atoms.
The boron atom in the ground state possesses \[1{s^2},2{s^2},2{p^1}\]electronic configuration. During the formation of \[BC{l_3}\]molecule, the one electron from \[2{s^2}\]is excited to the \[2p\]orbital to form \[1{s^2},2{s^1},2{p_x}^1,2{p_y}^1\]excited state electronic configuration. These three unpaired electrons are paired with the unpaired electron of chlorine and form three \[B - Cl\]bonds.
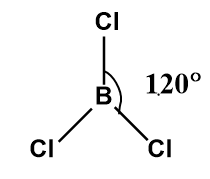
Image: Structure of boron trichloride (\[BC{l_3}\]).
Now, to determine the hybridization of \[BC{l_3}\]molecules two methods can be used.
(1) By using the following formula, we can predict the hybridization of any given molecule.
\[Hybridization(H) = \frac{{V + M - C + A}}{2}\] (Eq.1)
Whereas V=number of valence electrons on the central atom
M= a number of monovalent atoms
C= charge on the cation
A=charge on anion
Hence, \[Hybridization(H) = \frac{{3 + 3 - 0 + 0}}{2} = \frac{6}{2} = 3\]
The value of 3 will be equal to \[s{p^2}\] hybridization. Therefore, we can easily say that \[BC{l_3}\]has \[s{p^2}\]hybridization and trigonal planar structure with a bond angle \[120^\circ \].
(2) By counting the total number of sigma bonds:
By counting the total number of the sigma bond, we can exactly predict the type of hybridization:
If the sum of the sigma bond is two, then the hybridization will be \[s{p^{}}\].
If the sum of the sigma bond is three, then the hybridization will be \[s{p^2}\].
If the sum of the sigma bond is four, then the hybridization will be \[s{p^3}\].
If the sum of the sigma bond is five, then the hybridization will be \[s{p^3}d\].
If the sum of the sigma bond is six, then the hybridization will be \[s{p^3}{d^2}\].
Therefore, the \[BC{l_3}\]molecule has three sigma bonds. Hence the hybridization will be \[s{p^2}\].
Therefore from the above explanation we can say option (b) will be the correct option:
Note: In \[BC{l_3}\]molecule, the boron atom has \[ + 3\] oxidation state.
The \[BC{l_3}\]molecule is an electron-deficient species i.e., it can accept the electron pair from an electron donor moiety.
Borazine (\[{N_3}{B_3}{H_6}\]) is the compound of boron, which is called inorganic benzene.
Complete step by step solution:The \[BC{l_3}\]molecule is known as boron trichloride.
In the \[BC{l_3}\]molecule, the boron atom occupies a central position and is connected with three chlorine atoms.
The boron atom in the ground state possesses \[1{s^2},2{s^2},2{p^1}\]electronic configuration. During the formation of \[BC{l_3}\]molecule, the one electron from \[2{s^2}\]is excited to the \[2p\]orbital to form \[1{s^2},2{s^1},2{p_x}^1,2{p_y}^1\]excited state electronic configuration. These three unpaired electrons are paired with the unpaired electron of chlorine and form three \[B - Cl\]bonds.

Image: Structure of boron trichloride (\[BC{l_3}\]).
Now, to determine the hybridization of \[BC{l_3}\]molecules two methods can be used.
(1) By using the following formula, we can predict the hybridization of any given molecule.
\[Hybridization(H) = \frac{{V + M - C + A}}{2}\] (Eq.1)
Whereas V=number of valence electrons on the central atom
M= a number of monovalent atoms
C= charge on the cation
A=charge on anion
Hence, \[Hybridization(H) = \frac{{3 + 3 - 0 + 0}}{2} = \frac{6}{2} = 3\]
The value of 3 will be equal to \[s{p^2}\] hybridization. Therefore, we can easily say that \[BC{l_3}\]has \[s{p^2}\]hybridization and trigonal planar structure with a bond angle \[120^\circ \].
(2) By counting the total number of sigma bonds:
By counting the total number of the sigma bond, we can exactly predict the type of hybridization:
If the sum of the sigma bond is two, then the hybridization will be \[s{p^{}}\].
If the sum of the sigma bond is three, then the hybridization will be \[s{p^2}\].
If the sum of the sigma bond is four, then the hybridization will be \[s{p^3}\].
If the sum of the sigma bond is five, then the hybridization will be \[s{p^3}d\].
If the sum of the sigma bond is six, then the hybridization will be \[s{p^3}{d^2}\].
Therefore, the \[BC{l_3}\]molecule has three sigma bonds. Hence the hybridization will be \[s{p^2}\].
Therefore from the above explanation we can say option (b) will be the correct option:
Note: In \[BC{l_3}\]molecule, the boron atom has \[ + 3\] oxidation state.
The \[BC{l_3}\]molecule is an electron-deficient species i.e., it can accept the electron pair from an electron donor moiety.
Borazine (\[{N_3}{B_3}{H_6}\]) is the compound of boron, which is called inorganic benzene.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




