
The molecule with zero dipole moment is?
A. \[Cl{F_3}\]
B. \[Br{F_{_5}}\]
C. \[I{F_7}\]
D. \[ClF\]
Answer
233.1k+ views
Hint: Dipole moment is a vector quantity and it occurs when the charge is separated. The total amount of dipole movement can be calculated by multiplying the charge with the total distance between the charges.
Formula used<\b>: The dipole movement can be calculated by the given formula;
\[\mu = q.r\]
Where \[q\]stands for the magnitude of the charge that is separated, \[r\] represents the distance that is present between the charges and \[\mu \] represents the dipole moment.
The SI unit to measure the dipole moment is coulomb-metres(C-m).
The direction of the dipole moment is usually from negative to positive charge.
Complete step by step solution:
Dipole movement can occur between the two ions that are linked together by ionic bonds and between two or more atoms that are linked together by covalent bonds. So as per the question stated above, we need to check the net charge that is present:
1. \[Cl{F_3}\]
The structural diagram of \[Cl{F_3}\] is as follows:
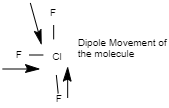
Image source: Chemdraw
Since three dipoles are present in the T-shaped molecule which do not cancel out each other, therefore the molecule of \[Cl{F_3}\] is polar i.e. the dipole moment is not zero.
2. \[Br{F_3}\]
The structural formula of \[Br{F_3}\] is as follows:
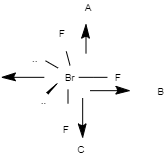
Image source: Chemdraw
As we can see here, in this T-shaped molecule, the dipole moment does not cancel out each other. There is a huge difference in the electronegativity of fluorine and bromine atoms. This is a highly polar molecule that is often used as a fluorinating agent. So the dipole moment is not zero.
3. \[I{F_7}\]
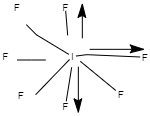
Image source: Chemdraw
Out of seven, 5 fluorine molecules are placed together in a single plane and their total magnitude and magnitude of the opposite fluorine atoms cancel out each other. Hence, they are non-polar and their resultant dipole moment is zero.
4. \[ClF\]
The structural formula of \[ClF\] is as follows;

Image source: Chemdraw
As per the chemical structure above, we can observe that it has a linear geometry and these are two different elements with different electronegativities. Hence the molecule mentioned above is polar and it has a non-zero dipole moment.
Hence, option C is the correct answer
Note: Molecules may or may not have a polar bond to have a net dipole moment. Many molecules have a polar bond and still have a zero-dipole moment. This is true when the structural formula of the molecules is symmetrical in which the individual dipole bonds cancel out each other.
Formula used<\b>: The dipole movement can be calculated by the given formula;
\[\mu = q.r\]
Where \[q\]stands for the magnitude of the charge that is separated, \[r\] represents the distance that is present between the charges and \[\mu \] represents the dipole moment.
The SI unit to measure the dipole moment is coulomb-metres(C-m).
The direction of the dipole moment is usually from negative to positive charge.
Complete step by step solution:
Dipole movement can occur between the two ions that are linked together by ionic bonds and between two or more atoms that are linked together by covalent bonds. So as per the question stated above, we need to check the net charge that is present:
1. \[Cl{F_3}\]
The structural diagram of \[Cl{F_3}\] is as follows:

Image source: Chemdraw
Since three dipoles are present in the T-shaped molecule which do not cancel out each other, therefore the molecule of \[Cl{F_3}\] is polar i.e. the dipole moment is not zero.
2. \[Br{F_3}\]
The structural formula of \[Br{F_3}\] is as follows:

Image source: Chemdraw
As we can see here, in this T-shaped molecule, the dipole moment does not cancel out each other. There is a huge difference in the electronegativity of fluorine and bromine atoms. This is a highly polar molecule that is often used as a fluorinating agent. So the dipole moment is not zero.
3. \[I{F_7}\]

Image source: Chemdraw
Out of seven, 5 fluorine molecules are placed together in a single plane and their total magnitude and magnitude of the opposite fluorine atoms cancel out each other. Hence, they are non-polar and their resultant dipole moment is zero.
4. \[ClF\]
The structural formula of \[ClF\] is as follows;

Image source: Chemdraw
As per the chemical structure above, we can observe that it has a linear geometry and these are two different elements with different electronegativities. Hence the molecule mentioned above is polar and it has a non-zero dipole moment.
Hence, option C is the correct answer
Note: Molecules may or may not have a polar bond to have a net dipole moment. Many molecules have a polar bond and still have a zero-dipole moment. This is true when the structural formula of the molecules is symmetrical in which the individual dipole bonds cancel out each other.
Recently Updated Pages
Know The Difference Between Fluid And Liquid

Types of Solutions in Chemistry: Explained Simply

Difference Between Crystalline and Amorphous Solid: Table & Examples

Hess Law of Constant Heat Summation: Definition, Formula & Applications

Disproportionation Reaction: Definition, Example & JEE Guide

JEE General Topics in Chemistry Important Concepts and Tips

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




