
The increasing order of stability of the following free radicals is.
(A) \[{{(C{{H}_{3}})}_{2}}\overset{\bullet }{\mathop{C}}\,H<{{(C{{H}_{3}})}_{3}}\overset{\bullet }{\mathop{C}}\,<{{({{C}_{6}}{{H}_{5}})}_{2}}\overset{\bullet }{\mathop{C}}\,H<{{({{C}_{6}}{{H}_{5}})}_{3}}\overset{\bullet }{\mathop{C}}\,\]
(B) \[{{({{C}_{6}}{{H}_{5}})}_{3}}\overset{\bullet }{\mathop{C}}\,<{{({{C}_{6}}{{H}_{5}})}_{2}}\overset{\bullet }{\mathop{C}}\,H<{{(C{{H}_{3}})}_{3}}\overset{\bullet }{\mathop{C}}\,<{{(C{{H}_{3}})}_{2}}\overset{\bullet }{\mathop{C}}\,H\]
(C) \[{{({{C}_{6}}{{H}_{5}})}_{2}}\overset{\bullet }{\mathop{C}}\,H<{{({{C}_{6}}{{H}_{5}})}_{3}}\overset{\bullet }{\mathop{C}}\,<{{(C{{H}_{3}})}_{3}}\overset{\bullet }{\mathop{C}}\,<{{(C{{H}_{3}})}_{2}}\overset{\bullet }{\mathop{C}}\,H\]
(D) \[{{(C{{H}_{3}})}_{2}}\overset{\bullet }{\mathop{C}}\,H<{{(C{{H}_{3}})}_{3}}\overset{\bullet }{\mathop{C}}\,<{{({{C}_{6}}{{H}_{5}})}_{3}}\overset{\bullet }{\mathop{C}}\,<{{({{C}_{6}}{{H}_{5}})}_{2}}\overset{\bullet }{\mathop{C}}\,H\]
Answer
233.1k+ views
Hint: In the stabilization of free radicals alpha hydrogens are going to play a big role. The hydrogens which are present on alpha carbon in an organic molecule are called alpha hydrogens. We can see the alpha hydrogen clearly from the below image.
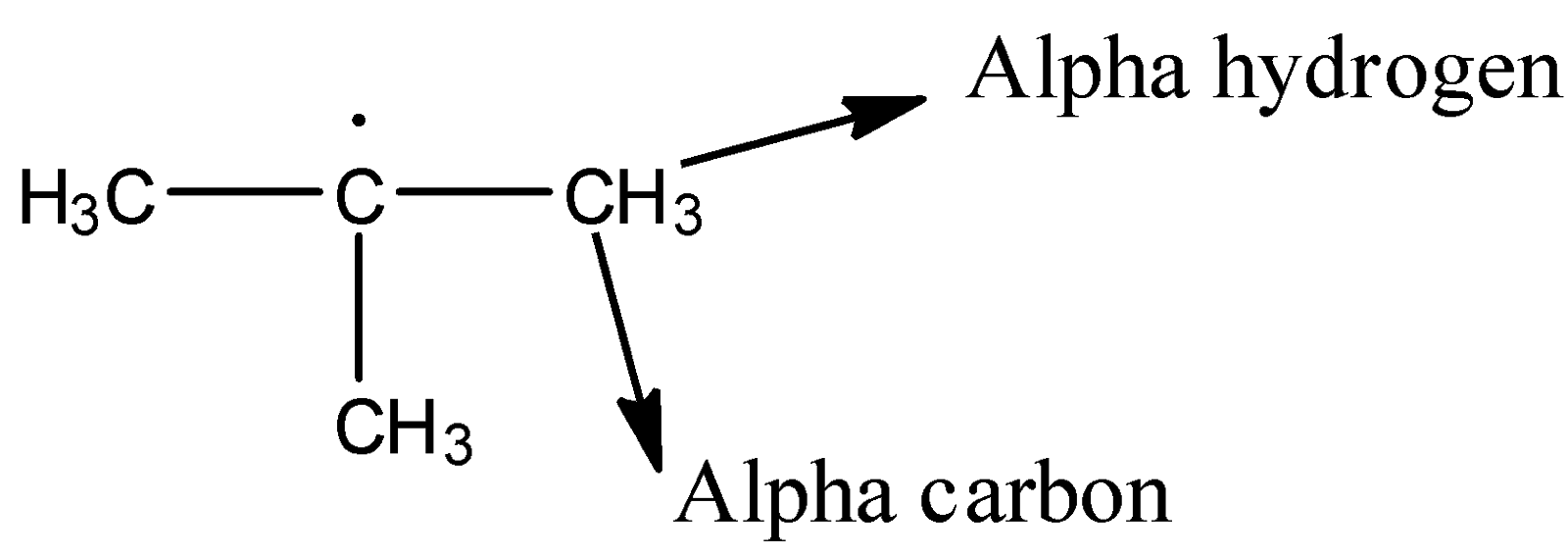
Complete step by step solution:
-We have to find the increasing order of stability of the given free radicals.
-Before going to find the stability of free radicals, we should know the factors that make free radicals more stable.
-Hyper conjugation and resonance are the main factors that decide which free radicals are more stable.
-In given options, we can see clearly that there are alkyl free radicals and phenyl free radicals.
-The given free radicals in the options are as follows.
\[{{(C{{H}_{3}})}_{2}}\overset{\bullet }{\mathop{C}}\,H,{{(C{{H}_{3}})}_{3}}\overset{\bullet }{\mathop{C}}\,,{{({{C}_{6}}{{H}_{5}})}_{2}}\overset{\bullet }{\mathop{C}}\,H,{{({{C}_{6}}{{H}_{5}})}_{3}}\overset{\bullet }{\mathop{C}}\,\]
-If an alkyl group has more number of alpha hydrogens then it shows more number of hyperconjugation structures.
-The order of stability of alkyl free radicals are as follows.
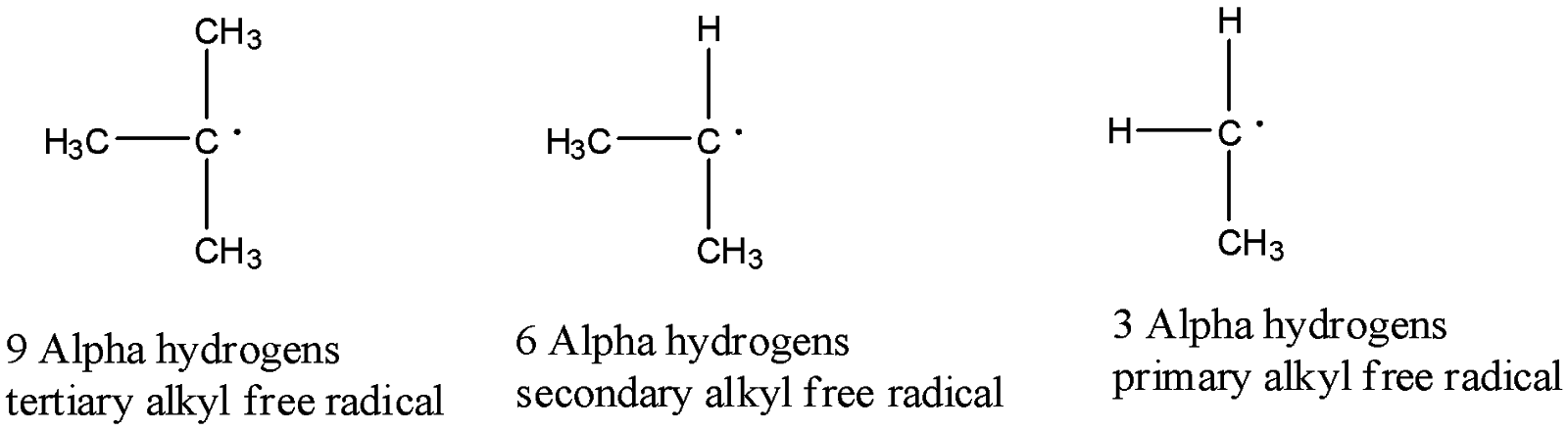
-Tertiary alkyl free radicals have 9 alpha hydrogens then it shows 9 hyperconjugation structures, secondary alkyl free radicals has 6 alpha hydrogens then it shows 6 hyperconjugation structures, and primary free radicals have 3 alpha hydrogens then it shows 3 hyperconjugation structures.
-Therefore in the given options there is one tertiary alkyl free radical and one secondary alkyl free radical. They are as follows
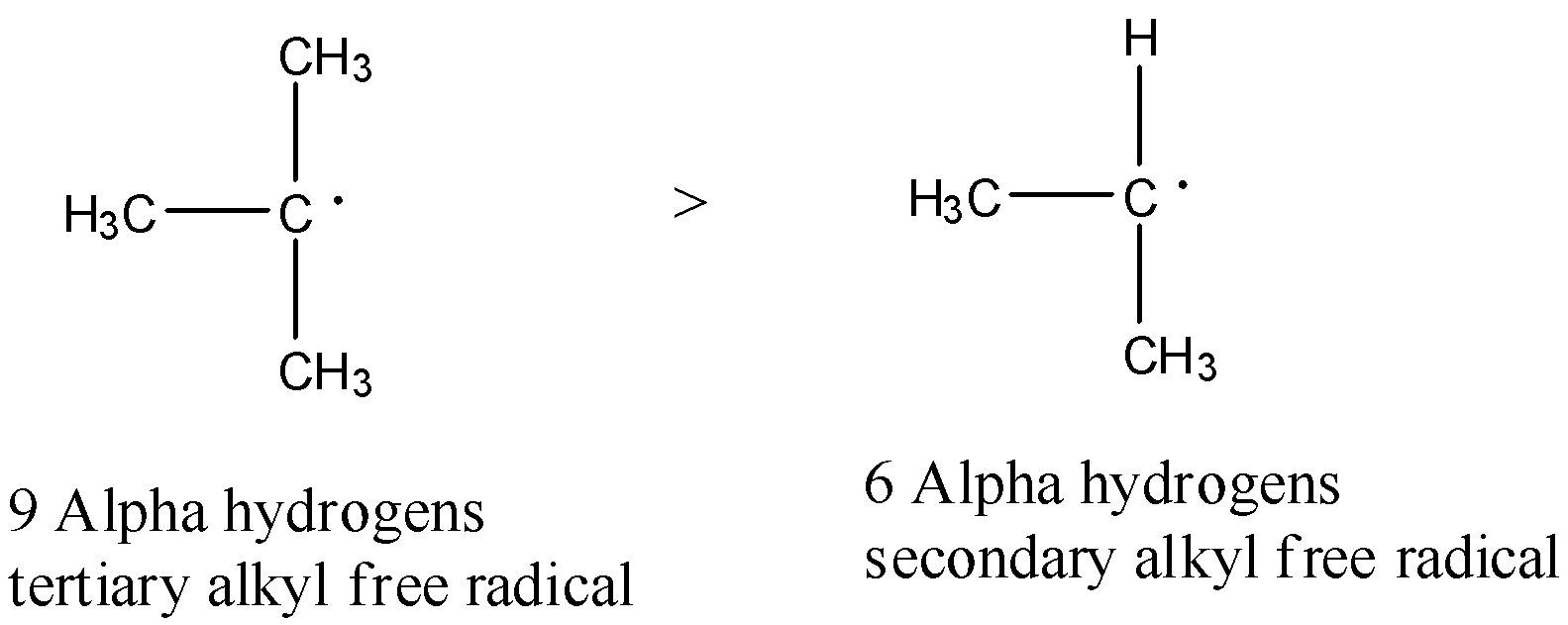
-Tertiary alkyl free radical is more stable than secondary alkyl free radical.
- Now coming to phenyl free radicals.
-The given phenyl free radicals in the options are as follows.
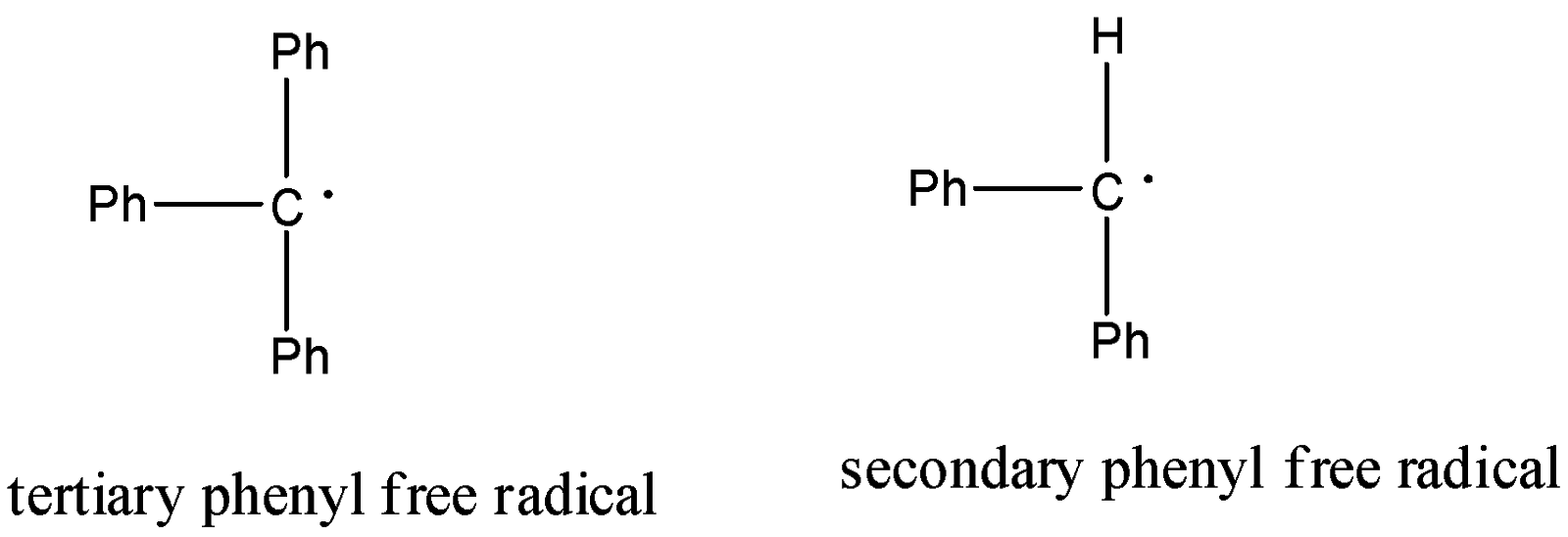
-Resonance is also a factor affecting the stabilization of free radicals.
-We know that phenyl groups also show resonance structures (hyperconjugation structures), the order of stability of phenyl free radicals are as follows.

-But the stability of phenyl free radicals are more stable than the alkyl free radicals because phenyl free radicals show resonance.
-Resonance free radicals have a higher impact than the hyperconjugation in alkyl free radicals.
-Therefore, the order of increasing the stability of free radicals is as follows.
\[{{(C{{H}_{3}})}_{2}}\overset{\bullet }{\mathop{C}}\,H<{{(C{{H}_{3}})}_{3}}\overset{\bullet }{\mathop{C}}\,<{{({{C}_{6}}{{H}_{5}})}_{2}}\overset{\bullet }{\mathop{C}}\,H<{{({{C}_{6}}{{H}_{5}})}_{3}}\overset{\bullet }{\mathop{C}}\,\]
So, the correct option is (A)..
Note: Hyperconjugation structures are possible in alkyl free radicals and resonance structures are possible in phenyl free radicals. Phenyl free radicals are more stable than the alkyl free radicals.

Complete step by step solution:
-We have to find the increasing order of stability of the given free radicals.
-Before going to find the stability of free radicals, we should know the factors that make free radicals more stable.
-Hyper conjugation and resonance are the main factors that decide which free radicals are more stable.
-In given options, we can see clearly that there are alkyl free radicals and phenyl free radicals.
-The given free radicals in the options are as follows.
\[{{(C{{H}_{3}})}_{2}}\overset{\bullet }{\mathop{C}}\,H,{{(C{{H}_{3}})}_{3}}\overset{\bullet }{\mathop{C}}\,,{{({{C}_{6}}{{H}_{5}})}_{2}}\overset{\bullet }{\mathop{C}}\,H,{{({{C}_{6}}{{H}_{5}})}_{3}}\overset{\bullet }{\mathop{C}}\,\]
-If an alkyl group has more number of alpha hydrogens then it shows more number of hyperconjugation structures.
-The order of stability of alkyl free radicals are as follows.

-Tertiary alkyl free radicals have 9 alpha hydrogens then it shows 9 hyperconjugation structures, secondary alkyl free radicals has 6 alpha hydrogens then it shows 6 hyperconjugation structures, and primary free radicals have 3 alpha hydrogens then it shows 3 hyperconjugation structures.
-Therefore in the given options there is one tertiary alkyl free radical and one secondary alkyl free radical. They are as follows

-Tertiary alkyl free radical is more stable than secondary alkyl free radical.
- Now coming to phenyl free radicals.
-The given phenyl free radicals in the options are as follows.

-Resonance is also a factor affecting the stabilization of free radicals.
-We know that phenyl groups also show resonance structures (hyperconjugation structures), the order of stability of phenyl free radicals are as follows.

-But the stability of phenyl free radicals are more stable than the alkyl free radicals because phenyl free radicals show resonance.
-Resonance free radicals have a higher impact than the hyperconjugation in alkyl free radicals.
-Therefore, the order of increasing the stability of free radicals is as follows.
\[{{(C{{H}_{3}})}_{2}}\overset{\bullet }{\mathop{C}}\,H<{{(C{{H}_{3}})}_{3}}\overset{\bullet }{\mathop{C}}\,<{{({{C}_{6}}{{H}_{5}})}_{2}}\overset{\bullet }{\mathop{C}}\,H<{{({{C}_{6}}{{H}_{5}})}_{3}}\overset{\bullet }{\mathop{C}}\,\]
So, the correct option is (A)..
Note: Hyperconjugation structures are possible in alkyl free radicals and resonance structures are possible in phenyl free radicals. Phenyl free radicals are more stable than the alkyl free radicals.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




