
The electron configuration for Potassium in the ground state:
(A)- $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}$
(B)- $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}4{{s}^{2}}$
(C)- $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}4{{s}^{1}}$
(D)- $1{{s}^{2}}$
(E)- $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{p}^{1}}$
Answer
233.1k+ views
Hint: Potassium in the ground state has 19 electrons and the shell structure is 2,8,8,1. To fill electrons in the orbitals there are some rules we must keep in mind namely Hund’s rule and Aufbau principle.
Complete step by step solution:
> In order to write the Potassium electron configuration we first need to know the number of electrons for the K atom. When we write the configuration we'll put all 19 electrons in orbitals around the nucleus of the Potassium atom.
> We need to distribute 19 electrons, knowing that there can be
- 2 in an s-orbital
- 6 in a p-orbital (2 in ${{p}_{x}}$, 2 in ${{p}_{y}}$ and 2 in ${{p}_{z}}$)
- 10 in a d-orbital
> We won’t need f-orbitals before atomic number 58.
The energy of an electron in a single electron atom can be determined by the principal quantum number. Thus, the order of increase in energy of various orbitals is given as, 1s < 2s = 2p < 3s = 3p = 3d <4s = 4p = 4d= 4f. But a multiple electron system (n+l) rule involving principal and azimuthal quantum numbers is followed although we do not need to apply it here.
> In writing the electron configuration for Potassium the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Potassium go in the 2s orbital.
> The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the next two electrons in the 3s.
Since the 3s are now full we'll move to the 3p where we'll place the next six electrons.
We now shift to the 4s orbital where we place the remaining electron.
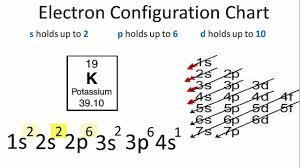
Therefore the Potassium electron configuration will be $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}4{{s}^{1}}$.
Hence, Option C is the correct answer.
Note: The electronic configuration provides an easy way for scientists to understand how electrons are arranged around the nucleus of an atom. This makes it easier to predict how atoms will interact to form chemical bonds.
Complete step by step solution:
> In order to write the Potassium electron configuration we first need to know the number of electrons for the K atom. When we write the configuration we'll put all 19 electrons in orbitals around the nucleus of the Potassium atom.
> We need to distribute 19 electrons, knowing that there can be
- 2 in an s-orbital
- 6 in a p-orbital (2 in ${{p}_{x}}$, 2 in ${{p}_{y}}$ and 2 in ${{p}_{z}}$)
- 10 in a d-orbital
> We won’t need f-orbitals before atomic number 58.
The energy of an electron in a single electron atom can be determined by the principal quantum number. Thus, the order of increase in energy of various orbitals is given as, 1s < 2s = 2p < 3s = 3p = 3d <4s = 4p = 4d= 4f. But a multiple electron system (n+l) rule involving principal and azimuthal quantum numbers is followed although we do not need to apply it here.
> In writing the electron configuration for Potassium the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Potassium go in the 2s orbital.
> The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the next two electrons in the 3s.
Since the 3s are now full we'll move to the 3p where we'll place the next six electrons.
We now shift to the 4s orbital where we place the remaining electron.

Therefore the Potassium electron configuration will be $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}4{{s}^{1}}$.
Hence, Option C is the correct answer.
Note: The electronic configuration provides an easy way for scientists to understand how electrons are arranged around the nucleus of an atom. This makes it easier to predict how atoms will interact to form chemical bonds.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




