
The correct statements among (a) to (b) are:
(a) Saline hydrides produce H2 gas when reacted with H2O.
(b) Reaction of LiAlH4 with BF3 leads to B2H6.
(c) PH3 and CH4 are electron - rich and electron precise hydrides, respectively.
(d) HF and CH4 are called molecular hydrides.
A. (c) and (d) only
B. (a), (b) and (c) only
C. (a), (b), (c) and (d)
D. (a), (c) and (d) only
Answer
232.8k+ views
Hint: Saline hydrides are compounds that form when hydrogen reacts with alkali metals or alkaline earth metals. Electron-rich hydrides are those that contain excess electrons as lone pairs on their central atom. These hydrides are formed by elements of groups 15, 16 and 17. Electron precise hydrides are those containing the exact number of valence electrons to form normal covalent bonds. They are usually formed by group 14 elements.
Complete Step by Step Solution:
To answer this question, we must go through each statement individually and check its correctness.
Statement (a)
Saline hydrides are those that form when hydrogen reacts with s-block elements which are found in groups 1 and 2 of the periodic table. The group 1 and group 2 elements are less electronegative than hydrogen. Examples include sodium hydride (\[NaH\]) and lithium hydride (\[LiH\]).
A characteristic property of saline hydrides is that they react vigorously with water to produce pure hydrogen gas. For example, \[NaH(s) + {H_2}O(l) \to NaOH(aq) + {H_2}(g)\]. Thus, statement (a) is correct.
Statement (b)
Lithium aluminium hydride (\[LiAl{H_4}\]) does react with boron trifluoride (\[B{F_3}\]) to form diborane (\[{B_2}{H_6}\]).
\[3LiAl{H_4} + 4B{F_3} \to 2{B_2}{H_6} + 3LiF + 3Al{F_3}\]
Thus, statement (b) is correct.
Statement (c)
Electron precise hydrides contain the exact number of electrons to form a normal covalent bond. It means that the number of valence electrons is equal to the number of covalent bonds that the hydride can form. Electron precise hydrides can be formed by group 14 elements. In methane (\[C{H_4}\]), carbon’s valence shell electronic configuration is \[2{s^2}2{p^2}\]i.e., it has four valence electrons, and it forms four covalent bonds with hydrogens in methane. Therefore, methane is an electron-precise hydride.
Electron-rich hydrides possess a greater number of valence electrons than the number of covalent bonds they can form. These extra valence electrons are present on their central atoms as lone pairs. Electron-rich hydrides are formed by elements of groups 15, 16 and 17. Phosphane’s (\[P{H_3}\]) central atom is phosphorus which belongs to group 15 and its valence shell electronic configuration is \[3{s^2}3{p^3}\] i.e., it has 5 valence electrons. But, in phosphine, phosphorus forms only 3 covalent bonds with hydrogens. The 2 extra electrons are present as lone pairs of the phosphorus atom as shown below.
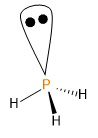
Image: Structure of Phosphine
Therefore, phosphane is an electron-rich hydride. Thus, statement (c) is correct.
Statement (d)
When hydrogen reacts with elements of similar electronegativity, the hydrides formed are called molecular hydrides. Molecular hydrides are also called covalent hydrides because the central atom and hydrogen are bonded by covalent bonds. Molecular hydrides are generally formed by non-metals (p-block elements). Hydrogen fluoride (\[HF\]) and methane (\[C{H_4}\]) are both examples of molecular hydrides. Thus, statement (d) is correct.
Thus, option C is the correct answer to this question.
Note: Ketone does not give a silver mirror test but alpha hydroxy ketone gives a silver mirror test. An alpha hydroxy ketone is a molecule having adjacent ketone and alcohol groups. Example: Glucose, tartaric acid.
Complete Step by Step Solution:
To answer this question, we must go through each statement individually and check its correctness.
Statement (a)
Saline hydrides are those that form when hydrogen reacts with s-block elements which are found in groups 1 and 2 of the periodic table. The group 1 and group 2 elements are less electronegative than hydrogen. Examples include sodium hydride (\[NaH\]) and lithium hydride (\[LiH\]).
A characteristic property of saline hydrides is that they react vigorously with water to produce pure hydrogen gas. For example, \[NaH(s) + {H_2}O(l) \to NaOH(aq) + {H_2}(g)\]. Thus, statement (a) is correct.
Statement (b)
Lithium aluminium hydride (\[LiAl{H_4}\]) does react with boron trifluoride (\[B{F_3}\]) to form diborane (\[{B_2}{H_6}\]).
\[3LiAl{H_4} + 4B{F_3} \to 2{B_2}{H_6} + 3LiF + 3Al{F_3}\]
Thus, statement (b) is correct.
Statement (c)
Electron precise hydrides contain the exact number of electrons to form a normal covalent bond. It means that the number of valence electrons is equal to the number of covalent bonds that the hydride can form. Electron precise hydrides can be formed by group 14 elements. In methane (\[C{H_4}\]), carbon’s valence shell electronic configuration is \[2{s^2}2{p^2}\]i.e., it has four valence electrons, and it forms four covalent bonds with hydrogens in methane. Therefore, methane is an electron-precise hydride.
Electron-rich hydrides possess a greater number of valence electrons than the number of covalent bonds they can form. These extra valence electrons are present on their central atoms as lone pairs. Electron-rich hydrides are formed by elements of groups 15, 16 and 17. Phosphane’s (\[P{H_3}\]) central atom is phosphorus which belongs to group 15 and its valence shell electronic configuration is \[3{s^2}3{p^3}\] i.e., it has 5 valence electrons. But, in phosphine, phosphorus forms only 3 covalent bonds with hydrogens. The 2 extra electrons are present as lone pairs of the phosphorus atom as shown below.

Image: Structure of Phosphine
Therefore, phosphane is an electron-rich hydride. Thus, statement (c) is correct.
Statement (d)
When hydrogen reacts with elements of similar electronegativity, the hydrides formed are called molecular hydrides. Molecular hydrides are also called covalent hydrides because the central atom and hydrogen are bonded by covalent bonds. Molecular hydrides are generally formed by non-metals (p-block elements). Hydrogen fluoride (\[HF\]) and methane (\[C{H_4}\]) are both examples of molecular hydrides. Thus, statement (d) is correct.
Thus, option C is the correct answer to this question.
Note: Ketone does not give a silver mirror test but alpha hydroxy ketone gives a silver mirror test. An alpha hydroxy ketone is a molecule having adjacent ketone and alcohol groups. Example: Glucose, tartaric acid.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




