
The compound used in the manufacture of terylene is
A. Ethylene
B. Vinyl chloride
C. Ethylene glycol
D. Adipic acid
Answer
233.1k+ views
Hint: Terylene is a synthetic polyester fibre based on terephthalic acid. It is light in weight and it is used in clothing, sheets, ropes.
Complete Step by Step Solution:
Terylene is manufactured by the condensation of ethylene glycol and Terephthalic acid (1,4-benzene dicarboxylic acid) with removal of water in this zinc acetate and antimony trioxide mixture is used as catalyst. The temperature required for this process is 151.85-degree Celsius to 201.85-degree Celsius. In polymerization many monomers react together and form polymers. Terylene is also known as Dacron.
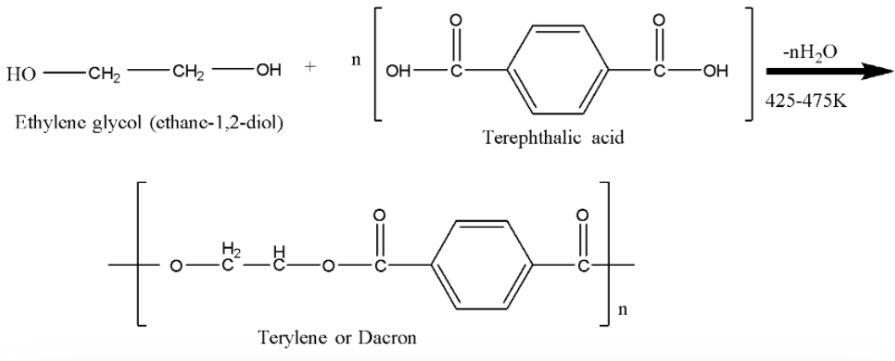
Here we can see that there are two monomers of ethylene glycol and terephthalic acid (1,4-benzene dicarboxylic acid) which are condensed and after the removal of water we get Terylene or Dacron. In this one linkage is present called ester linkage. Ester linkage is given below
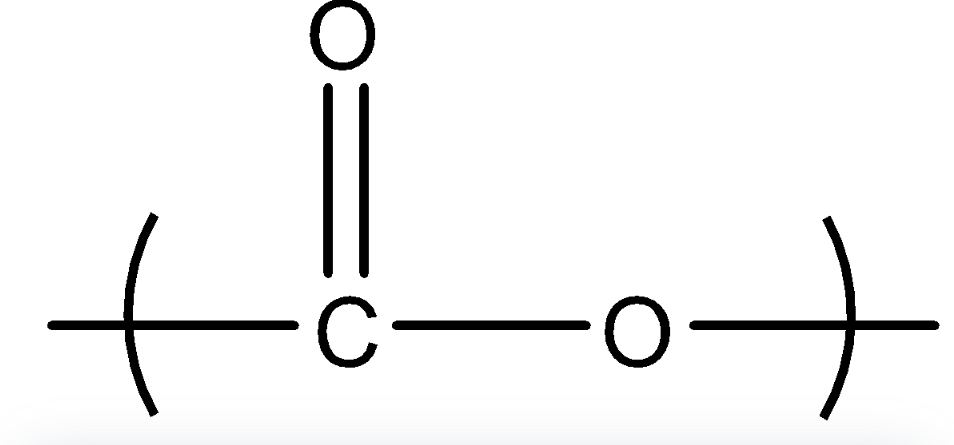
Image: Ester linkage
So, correct answer of the question is option (C) “Ethylene glycol”
Additional information:
Ethylene glycol and terephthalic acid is obtained from petroleum. This fibre was first created in 1941 by chemist J R Whinfield of Carington. The major disadvantage is they are not easily procurable by the yard. Terylene is majorly used in the textile industry to make many dresses. It is easily washed and dried quickly.it is used for laundry usage as an automatic clothing vacuum packing machine and also used for polyester tricot knit.
Note: Transesterification followed by polymerization gives terylene. Terylene is the strongest polymer. It is used to make non-woven needle punched carpet for exhibition.
Complete Step by Step Solution:
Terylene is manufactured by the condensation of ethylene glycol and Terephthalic acid (1,4-benzene dicarboxylic acid) with removal of water in this zinc acetate and antimony trioxide mixture is used as catalyst. The temperature required for this process is 151.85-degree Celsius to 201.85-degree Celsius. In polymerization many monomers react together and form polymers. Terylene is also known as Dacron.

Here we can see that there are two monomers of ethylene glycol and terephthalic acid (1,4-benzene dicarboxylic acid) which are condensed and after the removal of water we get Terylene or Dacron. In this one linkage is present called ester linkage. Ester linkage is given below

Image: Ester linkage
So, correct answer of the question is option (C) “Ethylene glycol”
Additional information:
Ethylene glycol and terephthalic acid is obtained from petroleum. This fibre was first created in 1941 by chemist J R Whinfield of Carington. The major disadvantage is they are not easily procurable by the yard. Terylene is majorly used in the textile industry to make many dresses. It is easily washed and dried quickly.it is used for laundry usage as an automatic clothing vacuum packing machine and also used for polyester tricot knit.
Note: Transesterification followed by polymerization gives terylene. Terylene is the strongest polymer. It is used to make non-woven needle punched carpet for exhibition.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




