
Propene when heated with chlorine at about 773K forms
A.
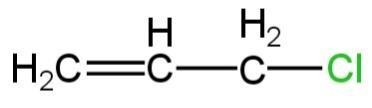
B.
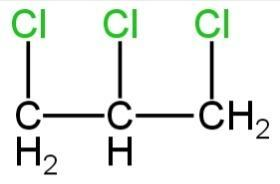
C.
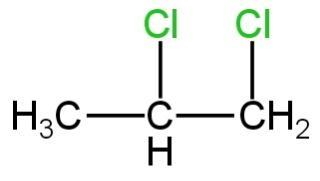
D. All of these
Answer
233.1k+ views
Hint: Halogenation of alkenes is the reaction of alkenes with chlorine, bromine, or iodine to produce a vicinal dihalide. This reaction happens in the existence of inert and non-nucleophilic solvents like methylene chloride, chloroform, or carbon tetrachloride.
Complete Step by Step Solution:
In this question, it is given that propene is undergoing a reaction with chlorine at about 773K. We have to find out the product it will form.
We know that propene will react with chlorine and will undergo halogenation.
It is because halogens can act as electrophiles to attack a double bond in an alkene.
A double bond depicts an area of electron density and thus acts as a nucleophile.
As chlorine approaches the double bond, electrons in the double bond are repelled by the electrons in the bromine molecule resulting in a polarization of the halogen bond.
This establishes a dipolar moment in the halogen molecule bond.
Then heterolytic bond division happens and one of the halogens acquires a positive charge and reacts as an electrophile.
This reaction ensues in two steps.
This reaction occurs at low temperatures.
But at high temperatures, chlorine undergoes homolytic cleavage to form chlorine free radicals.
This reaction occurs in three steps.
Initiation step
In the initial step of the addition, the Cl-Cl bond undergoes homolytic cleavage.
Chlorine free radicals are formed.
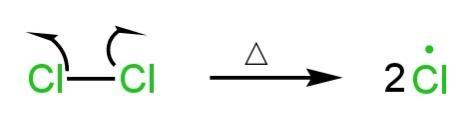
Image: Initiation step
Propagation step
One chlorine radical generated by homolytic cleavage in the initiation step eliminates allylic hydrogen from propane.
A radical intermediate is produced, which is stabilised by resonance.
So, allylic halogenation is preferred.
The intermediate radical then reacts with a chlorine molecule to produce the allylic chloride product which again forms the chlorine radical, which starts again the radical chain mechanism.
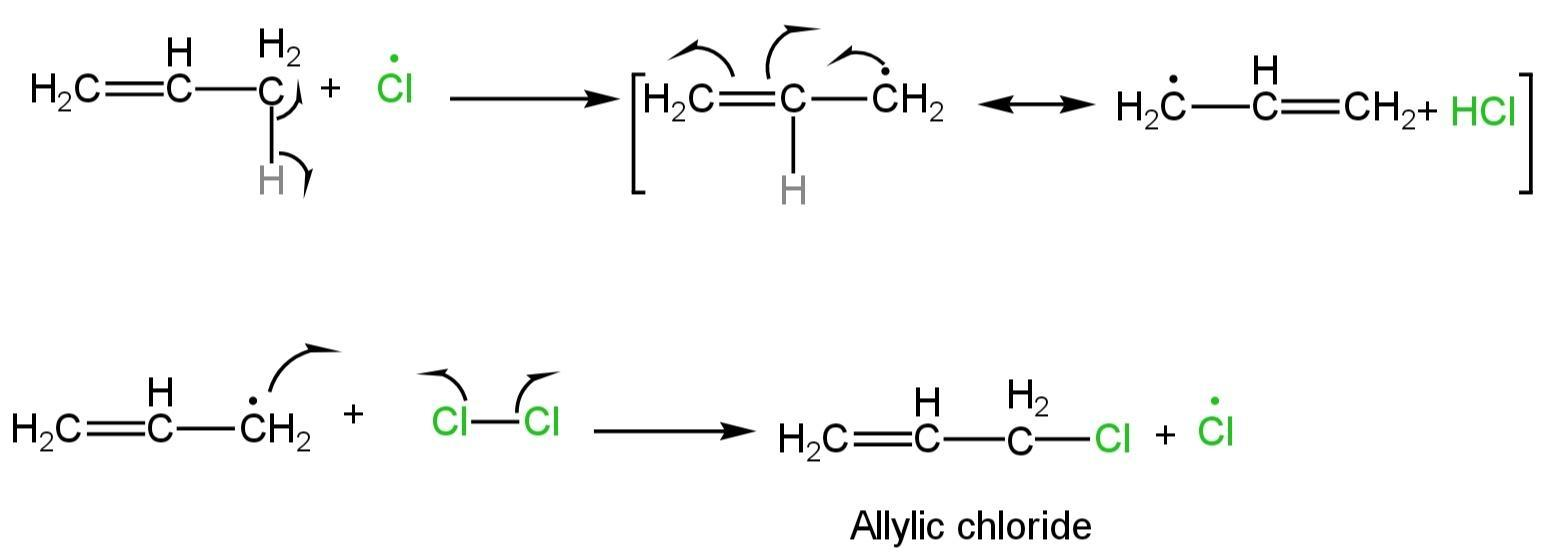
Image: Propagation step
Termination step
Termination happens by the combination of bromine radicals, one bromine radical with one allylic radical, and two allylic radicals.
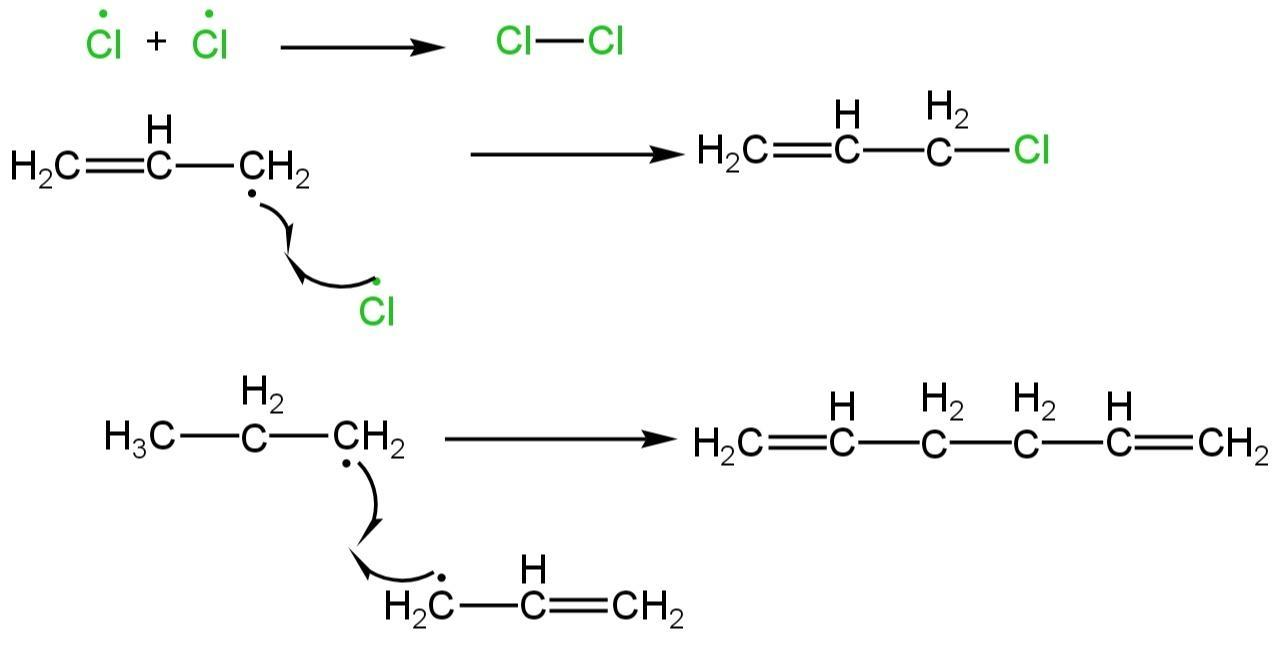
Image: Termination step
So, the product formed in this reaction is A.
So, option A is correct.
Note: Numerous routes exist for the halogenation of organic compounds, comprising free radical halogenation, ketone halogenation, electrophilic halogenation, and halogen addition reaction. The nature of the substrate specifies the pathway. Fluorination with elemental fluorine is extremely exothermic so highly specialised conditions and devices are employed.
Complete Step by Step Solution:
In this question, it is given that propene is undergoing a reaction with chlorine at about 773K. We have to find out the product it will form.
We know that propene will react with chlorine and will undergo halogenation.
It is because halogens can act as electrophiles to attack a double bond in an alkene.
A double bond depicts an area of electron density and thus acts as a nucleophile.
As chlorine approaches the double bond, electrons in the double bond are repelled by the electrons in the bromine molecule resulting in a polarization of the halogen bond.
This establishes a dipolar moment in the halogen molecule bond.
Then heterolytic bond division happens and one of the halogens acquires a positive charge and reacts as an electrophile.
This reaction ensues in two steps.
This reaction occurs at low temperatures.
But at high temperatures, chlorine undergoes homolytic cleavage to form chlorine free radicals.
This reaction occurs in three steps.
Initiation step
In the initial step of the addition, the Cl-Cl bond undergoes homolytic cleavage.
Chlorine free radicals are formed.
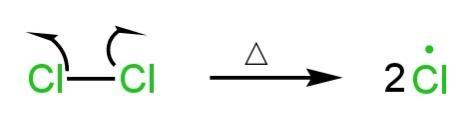
Image: Initiation step
Propagation step
One chlorine radical generated by homolytic cleavage in the initiation step eliminates allylic hydrogen from propane.
A radical intermediate is produced, which is stabilised by resonance.
So, allylic halogenation is preferred.
The intermediate radical then reacts with a chlorine molecule to produce the allylic chloride product which again forms the chlorine radical, which starts again the radical chain mechanism.

Image: Propagation step
Termination step
Termination happens by the combination of bromine radicals, one bromine radical with one allylic radical, and two allylic radicals.

Image: Termination step
So, the product formed in this reaction is A.
So, option A is correct.
Note: Numerous routes exist for the halogenation of organic compounds, comprising free radical halogenation, ketone halogenation, electrophilic halogenation, and halogen addition reaction. The nature of the substrate specifies the pathway. Fluorination with elemental fluorine is extremely exothermic so highly specialised conditions and devices are employed.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




