
What is the product X used for the following reaction:
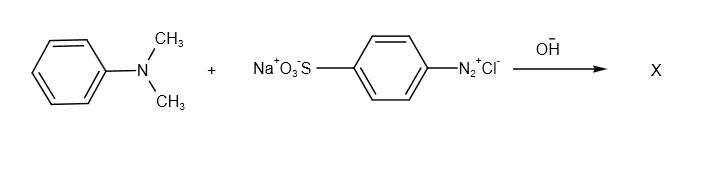
A. in protein estimation as an alternative to ninhydrin
B. as a food-grade colourant
C. in laboratory test for phenols
D. in acid-base titration as an indicator
Answer
233.1k+ views
Hint: Here, in this question, there is a reaction of N,N-dimethylaniline with an unstable diazonium salt in presence of a base. Acid is being released in this reaction.
Complete Step by Step Solution:
The chemical reaction between N,N-dimethylaniline and unstable diazonium salt in presence of base is as follows:
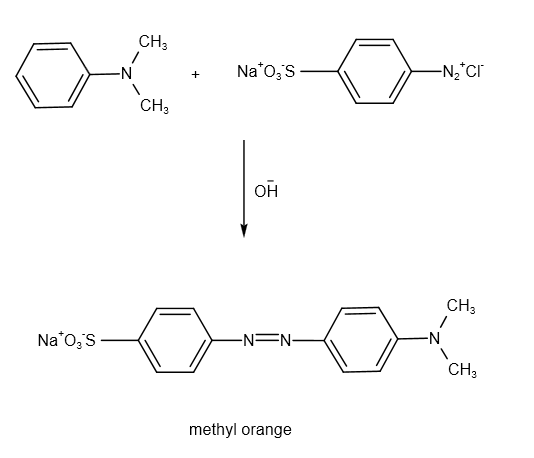
The reaction produces methyl orange.
In acid-base titration, methyl-orange is used as an indicator. Therefore, correct answer is option D.
Additional Information:
The methyl orange indicator has a pH of 3 to 5. In an acidic medium, methyl orange takes on a red colour, whereas, in a basic medium, it takes on a yellow colour. The colour of the solution shifted from red to yellow during the acid-base titration reaction. Because it is acid-sensitive, methyl orange is rarely used in textile applications.
Methyl orange is a brightly coloured chemical that is used in textile dyeing and printing. The colour change is caused by methyl orange absorbing light in the visible range of the electromagnetic spectrum. Methyl orange is a chromophore because it has an extensive conjugation system of delocalized electrons. The chromophore gives the compounds their colour.
Note: When it comes to identifying acid, bases, and other specific compounds, chemical indicators are known to have a certain range. The majority of the indicators will change colour, but some will change turbidity. As a result, the indicator has a transparent appearance. This change can be caused by the presence of a specific chemical or it can be a sign of neutralisation.
In the given solution, a Power of hydrogen (pH) indicator changes colour throughout a short range of pH values. There are a variety of pH indicators available, each of which displays a different colour and acts between specific pH ranges. Litmus paper is a classic example. When exposed to an acidic environment, blue litmus paper turns red, and red litmus paper turns blue when exposed to a basic environment.
Complete Step by Step Solution:
The chemical reaction between N,N-dimethylaniline and unstable diazonium salt in presence of base is as follows:

The reaction produces methyl orange.
In acid-base titration, methyl-orange is used as an indicator. Therefore, correct answer is option D.
Additional Information:
The methyl orange indicator has a pH of 3 to 5. In an acidic medium, methyl orange takes on a red colour, whereas, in a basic medium, it takes on a yellow colour. The colour of the solution shifted from red to yellow during the acid-base titration reaction. Because it is acid-sensitive, methyl orange is rarely used in textile applications.
Methyl orange is a brightly coloured chemical that is used in textile dyeing and printing. The colour change is caused by methyl orange absorbing light in the visible range of the electromagnetic spectrum. Methyl orange is a chromophore because it has an extensive conjugation system of delocalized electrons. The chromophore gives the compounds their colour.
Note: When it comes to identifying acid, bases, and other specific compounds, chemical indicators are known to have a certain range. The majority of the indicators will change colour, but some will change turbidity. As a result, the indicator has a transparent appearance. This change can be caused by the presence of a specific chemical or it can be a sign of neutralisation.
In the given solution, a Power of hydrogen (pH) indicator changes colour throughout a short range of pH values. There are a variety of pH indicators available, each of which displays a different colour and acts between specific pH ranges. Litmus paper is a classic example. When exposed to an acidic environment, blue litmus paper turns red, and red litmus paper turns blue when exposed to a basic environment.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




