
Primary nitro compounds when react with \[HN{{O}_{2}}\] from crystalline solids which on reaction with \[NaOH~\] gives
(A) Red solution
(B) Blue solution
(C) White precipitate
(D) Yellow coloration
Answer
233.4k+ views
Hint: Primary nitro compound is one in which the\[N{{O}_{2}}\] group is attached to one carbon which is further bonded with one alkyl chain or an alkyl group such as\[RC{{H}_{2}}N{{O}_{2}}\]. The reaction of this primary nitro compound with nitrous (\[HN{{O}_{2}}\]) results in releases of water molecules to give nitrolic acid. Again nitrolic acid reacts with \[NaOH~\](caustic soda) to release water molecules to give a soluble sodium salt.
Complete Step by Step Solution:
Now let us take a primary nitro alkyl compound, nitro ethane (such as\[C{{H}_{3}}C{{H}_{2}}N{{O}_{2}}\]which on reacting with nitrous reagent (\[HN{{O}_{2}}\]) gives nitrolic acid (double bond between carbon and nitrogen). In this, two hydrogen of first carbon from the nitro group makes the bond with one oxygen of nitrous to release water molecule such as
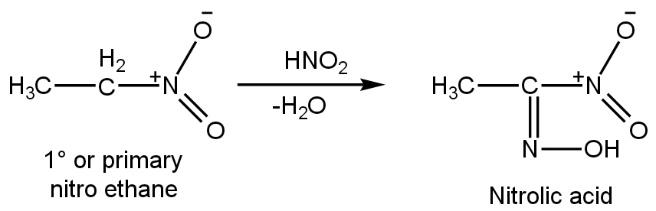
Now this nitrolic acid reacts with \[NaOH~\](caustic soda) to give soluble sodium salt because after reacting nitrolic acid with \[NaOH~\], the water molecule (H2O) gets liberated (OH group of \[NaOH~\]and hydrogen atom of NOH attached double boundedly with first carbon of nitrolic acid) and on the place of hydrogen atom (\[OH\]) sodium atom will attach and form sodium salt (\[{{O}^{-}}N{{a}^{+}}\]) such as
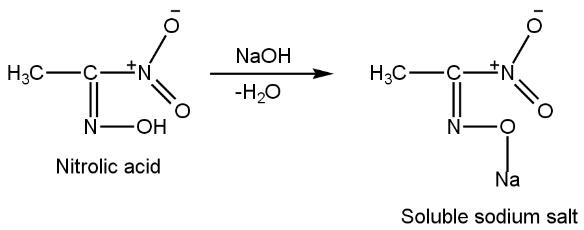
The soluble sodium salt formed is red in colour. Thus, the correct option is A.
Note: Primary nitro is one in which the \[N{{O}_{2}}\]group is attached to that carbon which is further attached to only one carbon (alkyl group) or with only one carbon chain (alkyl chain). If \[N{{O}_{2}}\]is attached to a carbon which further bonded with two carbon or with two carbon chains is known as a secondary or \[2{}^\circ \]nitro compound and if carbon is attached to three carbon further, then it is known as \[3{}^\circ \]nitro compound or tertiary nitro compound.
Complete Step by Step Solution:
Now let us take a primary nitro alkyl compound, nitro ethane (such as\[C{{H}_{3}}C{{H}_{2}}N{{O}_{2}}\]which on reacting with nitrous reagent (\[HN{{O}_{2}}\]) gives nitrolic acid (double bond between carbon and nitrogen). In this, two hydrogen of first carbon from the nitro group makes the bond with one oxygen of nitrous to release water molecule such as

Now this nitrolic acid reacts with \[NaOH~\](caustic soda) to give soluble sodium salt because after reacting nitrolic acid with \[NaOH~\], the water molecule (H2O) gets liberated (OH group of \[NaOH~\]and hydrogen atom of NOH attached double boundedly with first carbon of nitrolic acid) and on the place of hydrogen atom (\[OH\]) sodium atom will attach and form sodium salt (\[{{O}^{-}}N{{a}^{+}}\]) such as

The soluble sodium salt formed is red in colour. Thus, the correct option is A.
Note: Primary nitro is one in which the \[N{{O}_{2}}\]group is attached to that carbon which is further attached to only one carbon (alkyl group) or with only one carbon chain (alkyl chain). If \[N{{O}_{2}}\]is attached to a carbon which further bonded with two carbon or with two carbon chains is known as a secondary or \[2{}^\circ \]nitro compound and if carbon is attached to three carbon further, then it is known as \[3{}^\circ \]nitro compound or tertiary nitro compound.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




