
When phenol is distilled with Zn dust, the main product is:
(A) Biphenyl
(B) Benzene
(C) Benzaldehyde
(D) Phenolphthalein
Answer
536.9k+ views
Hint: Zinc dust acts as a reducing agent. Phenol undergoes reduction reaction in presence of zinc dust. Reduction reaction means the addition of electrons or removal of oxygen or addition of hydrogen.
Complete step by step answer:
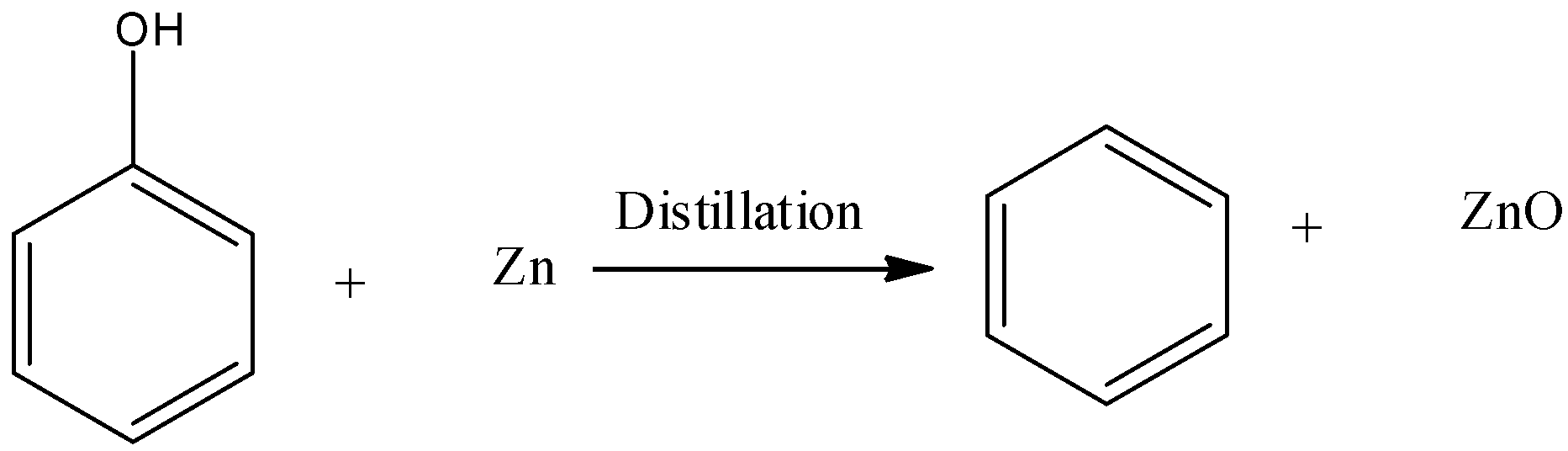
-We have to find the product when phenol is distilled with Zinc dust.
-Phenol undergoes a reduction reaction when heated with Zinc dust and forms benzene.
-It is one of the vital methods to prepare benzene.
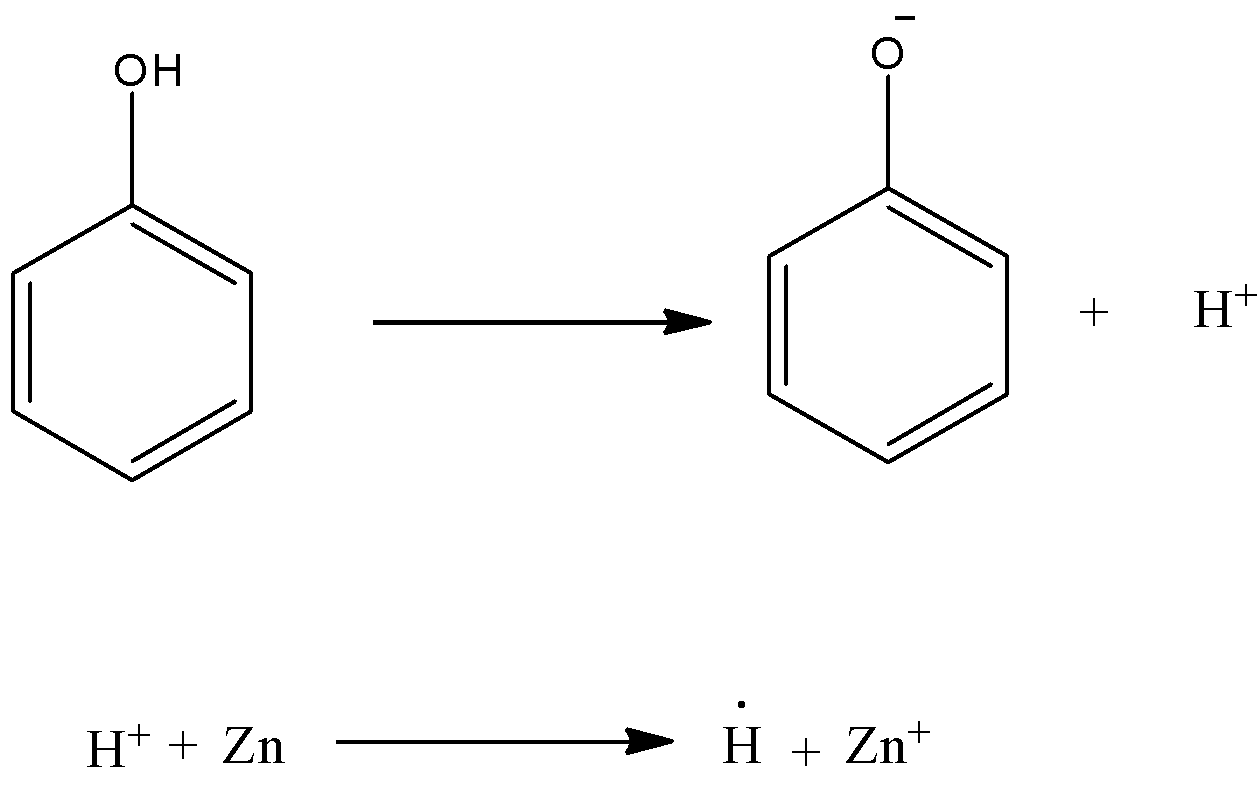
-In the above reaction, initially phenoxide ion is going to form by losing hydrogen.
-The hydrogen ion formed accepts electrons from zinc and converts into hydrogen radical.
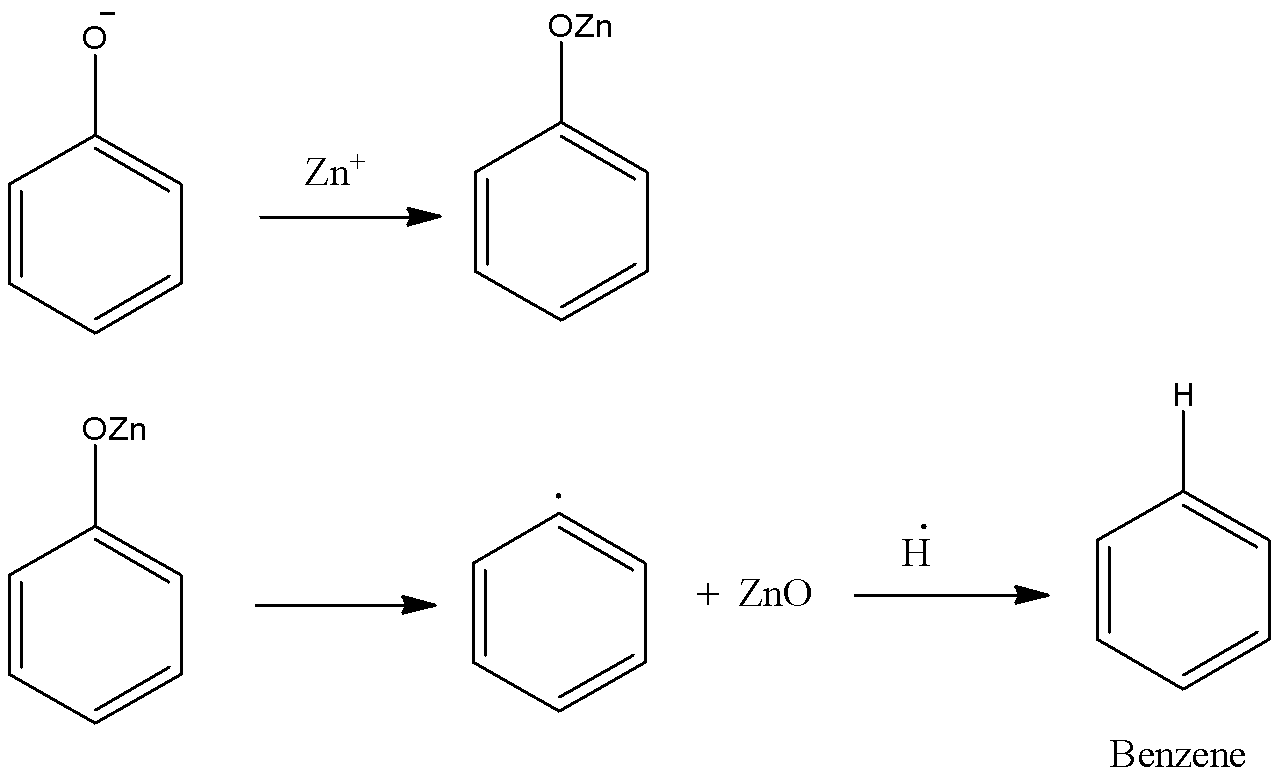
-Later the bond between carbon and oxygen breaks and generates phenyl radical, which combines with hydrogen radical and forms Benzene.
-The representation of the above discussion is as follows.
-Therefore when phenol is distilled with Zn dust, the main product is Benzene and zinc oxide also formed as a sub-product.
-Though the yield of benzene in this reaction is very less.
So, the correct option is B.
Additional information:
-Benzene is used as a solvent for different reactions in industries.
-Benzene is a carcinogenic solvent.
Note: Zinc dust at high temperatures acts as an excellent reducing agent. Zinc dust can be used as a reducing agent for the reduction of all aromatic compounds. The oxygen in the ketone functional group also can be replaced with hydrogen by using Zinc.
Complete step by step answer:
-We have to find the product when phenol is distilled with Zinc dust.
-Phenol undergoes a reduction reaction when heated with Zinc dust and forms benzene.
-It is one of the vital methods to prepare benzene.

-In the above reaction, initially phenoxide ion is going to form by losing hydrogen.
-The hydrogen ion formed accepts electrons from zinc and converts into hydrogen radical.

-Later the bond between carbon and oxygen breaks and generates phenyl radical, which combines with hydrogen radical and forms Benzene.
-The representation of the above discussion is as follows.

-Therefore when phenol is distilled with Zn dust, the main product is Benzene and zinc oxide also formed as a sub-product.
-Though the yield of benzene in this reaction is very less.
So, the correct option is B.
Additional information:
-Benzene is used as a solvent for different reactions in industries.
-Benzene is a carcinogenic solvent.
Note: Zinc dust at high temperatures acts as an excellent reducing agent. Zinc dust can be used as a reducing agent for the reduction of all aromatic compounds. The oxygen in the ketone functional group also can be replaced with hydrogen by using Zinc.
Recently Updated Pages
Types of Solutions in Chemistry: Explained Simply

JEE Main Mock Test 2025-26: Principles Related To Practical

JEE Main 2025-26 Organic Compounds Containing Nitrogen Mock Test

JEE Main 2025-26 Mock Test: Organic Compounds Containing Oxygen

JEE Main 2025-26 Redox Reactions & Electro Mock Test

JEE Main Solutions Mock Test 1-2 (2025-26): Free Practice & Answers

Trending doubts
JEE Main 2026: Session 1 Results Out and Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

Ideal and Non-Ideal Solutions Explained for Class 12 Chemistry

JEE Main Participating Colleges 2026 - A Complete List of Top Colleges

Understanding the Angle of Deviation in a Prism

Understanding Electromagnetic Waves and Their Importance

Hybridisation in Chemistry – Concept, Types & Applications

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

JEE Advanced 2026 - Exam Date (Released), Syllabus, Registration, Eligibility, Preparation, and More

NCERT Solutions For Class 12 Chemistry Chapter 2 Electrochemistry - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The D And F Block Elements - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules - 2025-26




