
In which of the following species the interatomic bond angle is \[109^\circ 28\prime \;\].
A.\[N{H_3},{\rm{ }}{\left( {B{F_4}} \right)^{ - 1}}\;\]
B.\[{\left( {N{H_4}} \right)^ + },{\rm{ }}B{F_3}\]
C.\[N{H_3},{\rm{ }}B{F_4}\]
D.\[{\left( {N{H_2}} \right)^{ - 1}},{\rm{ }}B{F_3}\]
Answer
233.4k+ views
Hint: Bond angle 109°28′
is demonstrated by a regular tetrahedral molecular geometry.
When the central metal atom having a tetrahedral shape has 4 bond pairs and no lone pairs, it will demonstrate this bond angle.
Complete step by step solution:The angle between two bonds arising from the same atom in a covalent species is comprehended as the bond angle.
The given bond angle is in species with regular tetrahedral geometry and they carry a hybridization of\[s{p^3}\].
For this, the steric number is 4. The steric number is the no.of atoms, groups, or lone pairs for the central metal atom.
For the given bond angle, there are generally 4 bonding pairs of electrons and no lone pairs.
So currently we have to study the provided molecules and attempt to find out the number of bond pairs in them.
Out of the given options, \[N{H_3},{\rm{ }}{\left( {B{F_4}} \right)^{ - 1}}\;\]both have interatomic bond angles of \[109^\circ 28\prime \;\].
\[N{H_3}\;\]
There is an N atom which is the central atom. It has 5 valence electrons.
Three electrons undergo bond formation with each of the three hydrogen atoms and the rest lone pair of electrons is there.
So, there are three bond pairs and one lone pair.
So, it has a pyramidal structure due to the repulsion between bond pairs and the lone pair.
So, the bond angle is \[109^\circ 28\prime \;\].
\[{\left( {B{F_4}} \right)^{ - 1}}\;\]
The shape is tetrahedral and, in the molecule, there are four bond pairs and zero lone pairs.
There are four B-F bonds.
So, the bond angle is \[109^\circ 28\prime \;\].
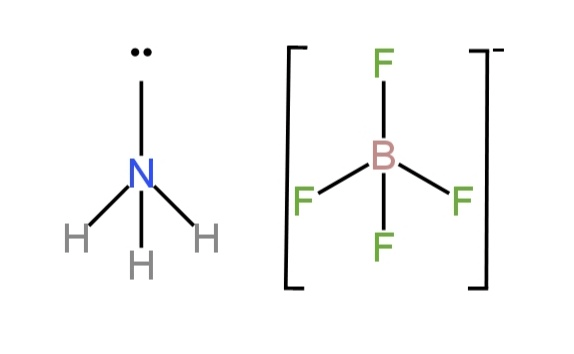
Image: Structure of \[N{H_3},{\rm{ }}{\left( {B{F_4}} \right)^{ - 1}}\;\]
So, option A is correct.
Note: Ammonia is one of the central components in a ton of household cleaning products.
It is utilized as a cleaning agent and can be utilized to peel off stains from mirrors, tubs, sinks, windows, and more. Some other benefits are antimicrobial agents or antiseptics, and ammonia is also utilized as a fuel.
is demonstrated by a regular tetrahedral molecular geometry.
When the central metal atom having a tetrahedral shape has 4 bond pairs and no lone pairs, it will demonstrate this bond angle.
Complete step by step solution:The angle between two bonds arising from the same atom in a covalent species is comprehended as the bond angle.
The given bond angle is in species with regular tetrahedral geometry and they carry a hybridization of\[s{p^3}\].
For this, the steric number is 4. The steric number is the no.of atoms, groups, or lone pairs for the central metal atom.
For the given bond angle, there are generally 4 bonding pairs of electrons and no lone pairs.
So currently we have to study the provided molecules and attempt to find out the number of bond pairs in them.
Out of the given options, \[N{H_3},{\rm{ }}{\left( {B{F_4}} \right)^{ - 1}}\;\]both have interatomic bond angles of \[109^\circ 28\prime \;\].
\[N{H_3}\;\]
There is an N atom which is the central atom. It has 5 valence electrons.
Three electrons undergo bond formation with each of the three hydrogen atoms and the rest lone pair of electrons is there.
So, there are three bond pairs and one lone pair.
So, it has a pyramidal structure due to the repulsion between bond pairs and the lone pair.
So, the bond angle is \[109^\circ 28\prime \;\].
\[{\left( {B{F_4}} \right)^{ - 1}}\;\]
The shape is tetrahedral and, in the molecule, there are four bond pairs and zero lone pairs.
There are four B-F bonds.
So, the bond angle is \[109^\circ 28\prime \;\].

Image: Structure of \[N{H_3},{\rm{ }}{\left( {B{F_4}} \right)^{ - 1}}\;\]
So, option A is correct.
Note: Ammonia is one of the central components in a ton of household cleaning products.
It is utilized as a cleaning agent and can be utilized to peel off stains from mirrors, tubs, sinks, windows, and more. Some other benefits are antimicrobial agents or antiseptics, and ammonia is also utilized as a fuel.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




