
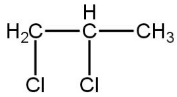
In the following reaction sequence:
give turbidity immediately
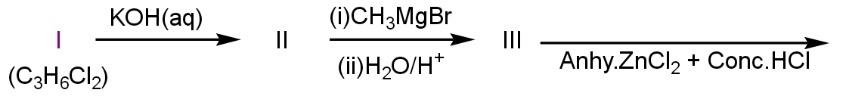
(A)
(B)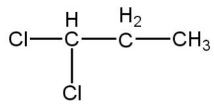
(C)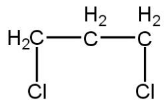
(D)
Answer
232.8k+ views
Hint: Turbidity in terms of the Lucas test is the measure to differentiate primary, secondary and tertiary alcohol when reacts with the Lucas reagent \[\left( Anhy.ZnC{{l}_{2}}+\text{ }Conc.HCl \right)\]. Turbidity (cloudiness) will immediately appear in the case of three-degree alcohol (tertiary). We need to reduce the turbidity of drinking water to get healthy purified water.
Complete Step by Step Solution:
Compound A on hydrolysis by \[KOH\text{ }\left( aq \right)\] gives compound \[propane-1,1-diol\]. This compound further releases a water molecule and after the attack of the Grignard reagent gives primary alcohol.
Compound B also gives primary alcohol after hydrolysis \[\left( give\text{ }propane-1,3-diol \right)\]and further reacts with the Grignard reagent.
Compound C which when reacting with aqueous KOH gets hydrolyzed and gives \[propane-2,2-diol\] (unstable) in step 1. This compound further releases a water molecule to give a ketone which is in equilibrium with the compound formed in the first step. This ketone, further attacked by Grignard reagent (negative part,\[C{{H}_{3}}\] of Grignard reagent attack ketonic carbon, positively charged) and oxygen (negatively charged)of ketone group get protonated in presence of water molecule to give the hydroxyl group (\[OH\]group) such as
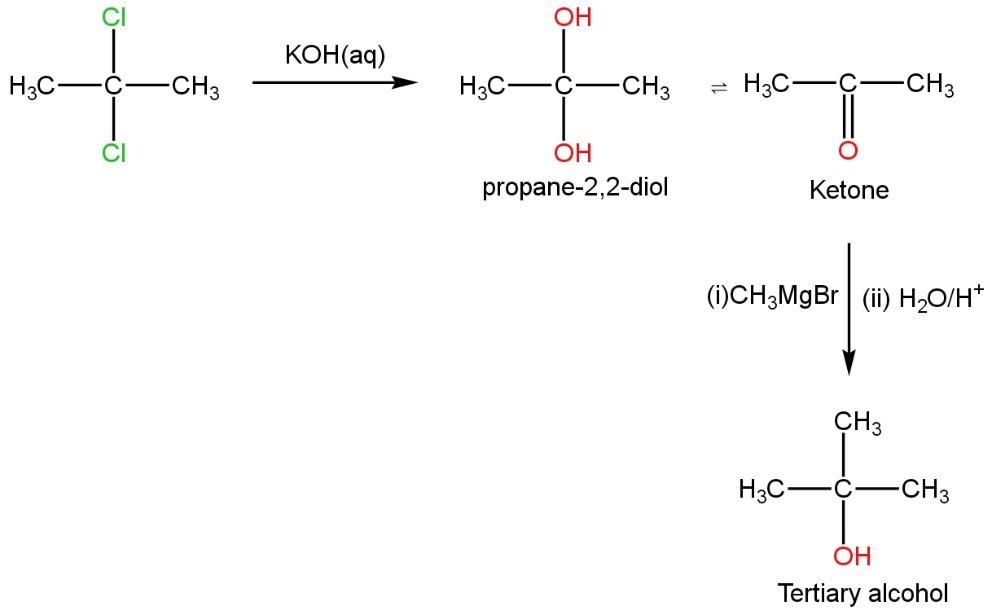
This results in the formation of three-degree alcohol (tertiary alcohol). This three-degree alcohol immediately gives turbidity under the action of Lucas’s reagent.
Thus, the correct option is C.
Note: Aqueous\[KOH\]is used to hydrolysis another compound as\[KOH\]is a strong nucleophile reagent. In any halogen-containing compound, halogens are good leaving groups and thus leave the compound due to its large size and high electronegativity value. As at least the compound, carbonation generated and thus attacked by\[O{{H}^{-}}\] (nucleophile), hydroxyl ion and hydrolysis take place. Grignard reagent molecular formula is\[RMgX\], where R is an alkyl group and X is a halogen. In any aqueous solution Grignard reagent breaks into negative (R) and positive (MgX) ions.
Complete Step by Step Solution:
Compound A on hydrolysis by \[KOH\text{ }\left( aq \right)\] gives compound \[propane-1,1-diol\]. This compound further releases a water molecule and after the attack of the Grignard reagent gives primary alcohol.
Compound B also gives primary alcohol after hydrolysis \[\left( give\text{ }propane-1,3-diol \right)\]and further reacts with the Grignard reagent.
Compound C which when reacting with aqueous KOH gets hydrolyzed and gives \[propane-2,2-diol\] (unstable) in step 1. This compound further releases a water molecule to give a ketone which is in equilibrium with the compound formed in the first step. This ketone, further attacked by Grignard reagent (negative part,\[C{{H}_{3}}\] of Grignard reagent attack ketonic carbon, positively charged) and oxygen (negatively charged)of ketone group get protonated in presence of water molecule to give the hydroxyl group (\[OH\]group) such as

This results in the formation of three-degree alcohol (tertiary alcohol). This three-degree alcohol immediately gives turbidity under the action of Lucas’s reagent.
Thus, the correct option is C.
Note: Aqueous\[KOH\]is used to hydrolysis another compound as\[KOH\]is a strong nucleophile reagent. In any halogen-containing compound, halogens are good leaving groups and thus leave the compound due to its large size and high electronegativity value. As at least the compound, carbonation generated and thus attacked by\[O{{H}^{-}}\] (nucleophile), hydroxyl ion and hydrolysis take place. Grignard reagent molecular formula is\[RMgX\], where R is an alkyl group and X is a halogen. In any aqueous solution Grignard reagent breaks into negative (R) and positive (MgX) ions.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




