
In the diagram, section A represents:
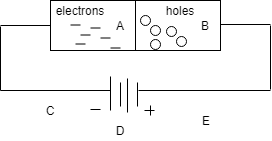
A) N-type germanium
B) P-type germanium
C) Anode
D) Diode
Answer
233.1k+ views
Hint: From figure when external source is applied then the charges get aligned and the excess of electrons gathers at one place and holes at the opposite side. Excess of electrons is N and holes are of P type.
Complete solution:
Step 1:
Before starting the solution let us know about N and P type semiconductor.
An N-type semiconductor is a type of material used in electronics. It is made by adding an impurity to a pure semiconductor such as silicon or germanium. The impurities used may be phosphorus, arsenic, antimony, bismuth or some other chemical element. They are called donor impurities.
A P-type semiconductor is a type of semiconductor. When the trivalent impurity is added to an intrinsic or pure semiconductor (silicon or germanium), then it is said to be a p-type semiconductor. Trivalent impurities such as Boron, Gallium, Indium, Aluminum etc are called acceptor impurity.
Step 2:
In N-type material there are electrons energy levels near the top of the band gap so that they can be easily excited into the conduction band. In P-type material, extra holes in the band gap allow excitation of valence band electrons, leaving mobile holes in the valence band.
So from the figure we can see that A has more number of electrons and B part has the maximum number of holes so A represents N-type Germanium.
Hence, option A is correct.
Additional information: Anode: The anode is the electrode where electricity moves into. The cathode is the electrode where electricity is given out or flows out of. The anode is usually the positive side.
Cathode: A cathode is the metallic electrode through which current flows out in a polarized electrical device. Cathodes get their name from cations (positively charged ions) and anodes from anions (negatively charged ions). In a device that uses electricity, the cathode is the negatively charged electrode.
Note: Semiconductors are especially made up of germanium and silicon because they have free electrons in their outer shell and hence offer conductivity. Germanium at a given temperature offers more free electrons than silicon. That’s why they are widely used in transistors and other electronic devices.
Complete solution:
Step 1:
Before starting the solution let us know about N and P type semiconductor.
An N-type semiconductor is a type of material used in electronics. It is made by adding an impurity to a pure semiconductor such as silicon or germanium. The impurities used may be phosphorus, arsenic, antimony, bismuth or some other chemical element. They are called donor impurities.
A P-type semiconductor is a type of semiconductor. When the trivalent impurity is added to an intrinsic or pure semiconductor (silicon or germanium), then it is said to be a p-type semiconductor. Trivalent impurities such as Boron, Gallium, Indium, Aluminum etc are called acceptor impurity.
Step 2:
In N-type material there are electrons energy levels near the top of the band gap so that they can be easily excited into the conduction band. In P-type material, extra holes in the band gap allow excitation of valence band electrons, leaving mobile holes in the valence band.
So from the figure we can see that A has more number of electrons and B part has the maximum number of holes so A represents N-type Germanium.
Hence, option A is correct.
Additional information: Anode: The anode is the electrode where electricity moves into. The cathode is the electrode where electricity is given out or flows out of. The anode is usually the positive side.
Cathode: A cathode is the metallic electrode through which current flows out in a polarized electrical device. Cathodes get their name from cations (positively charged ions) and anodes from anions (negatively charged ions). In a device that uses electricity, the cathode is the negatively charged electrode.
Note: Semiconductors are especially made up of germanium and silicon because they have free electrons in their outer shell and hence offer conductivity. Germanium at a given temperature offers more free electrons than silicon. That’s why they are widely used in transistors and other electronic devices.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding Uniform Acceleration in Physics

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Dual Nature of Radiation and Matter Class 12 Physics Chapter 11 CBSE Notes - 2025-26

Understanding the Electric Field of a Uniformly Charged Ring

JEE Advanced Weightage 2025 Chapter-Wise for Physics, Maths and Chemistry

Derivation of Equation of Trajectory Explained for Students

Understanding Electromagnetic Waves and Their Importance




