
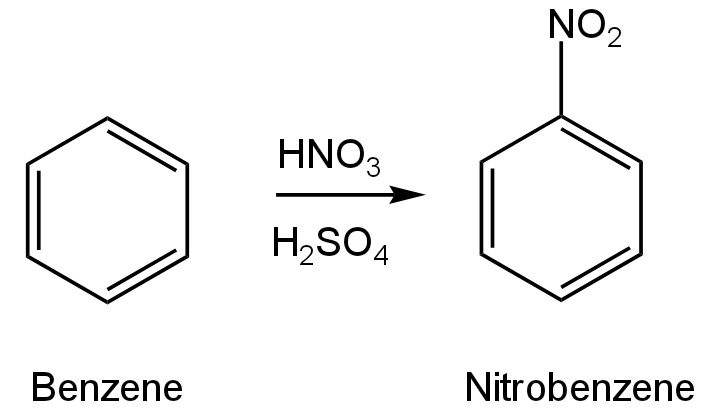
In the above reaction, 3.9 g of benzene on nitration gives 4.92g of nitrobenzene. The percentage yield of nitrobenzene in the above reaction is _____%. (Round off to the Nearest Integer). (Given atomic mass : C:\[12\] u, H: \[1\]u, O:\[16\]u, N:\[14\] u)
Answer
233.1k+ views
Hint: Carbon exists in a wide number of allotropic forms and diamond is one of them.vIt is the purest form of carbon. It is a huge three-dimensional polymer containing a large number of \[s{p^3}\] hybridised carbon atoms organised in a tetrahedral structure.
Formula used: Percentage yield \[ = \dfrac{{{\rm{Actual yield}}}}{{{\rm{Theoretical yield}}}}{\rm{ \times 100}}\]
Complete Step by Step Solution:
Benzene undergoes nitration by reacting with Nitric acid in the presence of Sulfuric acid.
The molecular mass of Benzene
= \[6\](molar mass of C)+\[6\](molar mass of H)
= (\[6 \times 12 + 6 \times 1\])\[{\rm{gmo}}{{\rm{l}}^{{\rm{ - 1}}}}\]
= \[{\rm{78 gmo}}{{\rm{l}}^{{\rm{ - 1}}}}\]
The chemical formula of Nitrobenzene is \[{{\rm{C}}_{\rm{6}}}{{\rm{H}}_{\rm{5}}}{\rm{N}}{{\rm{O}}_{\rm{2}}}\].
Its molecular mass
= \[6\](molar mass of C)+\[{\rm{5}}\](molar mass of H)+ molar mass of N+\[2\](molar mass of O)
= \[\left( {{\rm{6 \times 12 + 5 \times 1 + 14 + 2 \times 16}}} \right){\rm{g/mol}}\]
= \[{\rm{123g/mol\;\;\;\;\;\;\;\;\;\;\;\;\;}}\]
Here,1 mole of Benzene is used up in producing 1 mole of Nitrobenzene.
78g of Benzene is used up in producing 123g of Nitrobenzene.
1 g of Benzene is used up in producing \[\dfrac{{123}}{{78}}\]g of Nitrobenzene.
Hence,3.9 g of Benzene is used up in producing \[\dfrac{{123{\rm{ }}}}{{78}} \times 3.9\] g of Nitrobenzene.
So, 3.9 g of Benzene is used up in producing 6.15g of Nitrobenzene.
But according to the question, 4.92g of Nitrobenzene is formed.
So, the percentage yield
=\[\dfrac{{{\rm{4}}{\rm{.92g}}}}{{{\rm{6}}{\rm{.15g}}}}{\rm{(100)}}\].
\[ = 80\% \]
So, the percentage yield is\[80\% \] .
Note: While attempting the question, one must be careful while calculating the molecular mass of Benzene and Nitrobenzene. The chemical formula of these two compounds must be remembered. Percentage yield is the ratio of the actual yield to theoretical yield multiplied by 100. Here the actual yield is less than the theoretically predicted value which might be because of the loss of the reactants in forming products other than the given product.
Formula used: Percentage yield \[ = \dfrac{{{\rm{Actual yield}}}}{{{\rm{Theoretical yield}}}}{\rm{ \times 100}}\]
Complete Step by Step Solution:
Benzene undergoes nitration by reacting with Nitric acid in the presence of Sulfuric acid.
The molecular mass of Benzene
= \[6\](molar mass of C)+\[6\](molar mass of H)
= (\[6 \times 12 + 6 \times 1\])\[{\rm{gmo}}{{\rm{l}}^{{\rm{ - 1}}}}\]
= \[{\rm{78 gmo}}{{\rm{l}}^{{\rm{ - 1}}}}\]
The chemical formula of Nitrobenzene is \[{{\rm{C}}_{\rm{6}}}{{\rm{H}}_{\rm{5}}}{\rm{N}}{{\rm{O}}_{\rm{2}}}\].
Its molecular mass
= \[6\](molar mass of C)+\[{\rm{5}}\](molar mass of H)+ molar mass of N+\[2\](molar mass of O)
= \[\left( {{\rm{6 \times 12 + 5 \times 1 + 14 + 2 \times 16}}} \right){\rm{g/mol}}\]
= \[{\rm{123g/mol\;\;\;\;\;\;\;\;\;\;\;\;\;}}\]
Here,1 mole of Benzene is used up in producing 1 mole of Nitrobenzene.
78g of Benzene is used up in producing 123g of Nitrobenzene.
1 g of Benzene is used up in producing \[\dfrac{{123}}{{78}}\]g of Nitrobenzene.
Hence,3.9 g of Benzene is used up in producing \[\dfrac{{123{\rm{ }}}}{{78}} \times 3.9\] g of Nitrobenzene.
So, 3.9 g of Benzene is used up in producing 6.15g of Nitrobenzene.
But according to the question, 4.92g of Nitrobenzene is formed.
So, the percentage yield
=\[\dfrac{{{\rm{4}}{\rm{.92g}}}}{{{\rm{6}}{\rm{.15g}}}}{\rm{(100)}}\].
\[ = 80\% \]
So, the percentage yield is\[80\% \] .
Note: While attempting the question, one must be careful while calculating the molecular mass of Benzene and Nitrobenzene. The chemical formula of these two compounds must be remembered. Percentage yield is the ratio of the actual yield to theoretical yield multiplied by 100. Here the actual yield is less than the theoretically predicted value which might be because of the loss of the reactants in forming products other than the given product.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




