
In Carius’ method of estimation of halogen, 0.172 g of an organic compound showed the presence of 0.08 g of bromine. Which of these is the correct structure of the compound?
A.
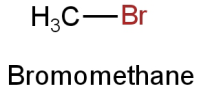
B.
C.
D.
Answer
233.1k+ views
Hint: Carius method is a procedure used to estimate the percentage of halogen in an organic compound.
In this method, a halogen-containing organic compound is heated in the presence of silver nitrate.
Formula Used:
Percentage of halogen =
\[\dfrac{{{\rm{the atomic mass of bromine}}}}{{{\rm{mass of the organic compound}}}}{\rm{ }}\].×100
Complete Step by Step Solution:
The mass of the organic compound = 0.172g
Mass of bromine present in the organic compound = 0.08g
So, the percentage of bromine =\[\dfrac{{0.08}}{{0.172}}(100)\] \[ = 46.51\% \].
The atomic mass of bromine = 80g.
A.
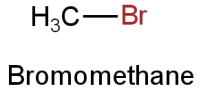
Image: Option A
This is bromomethane.
Molar mass
\[{\rm{ = [12 + 3(1) + 80]g}}\]
\[{\rm{ = 95g}}\]
The molar mass of bromoethane is 95g.
There is one bromine atom.
So, it contains 80g of bromine.
So, the percentage of bromine
\[ = \dfrac{{80}}{{95}} \times 100 = 84.21\% \]
This doesn't resemble the percentage of bromine in the given organic compound.
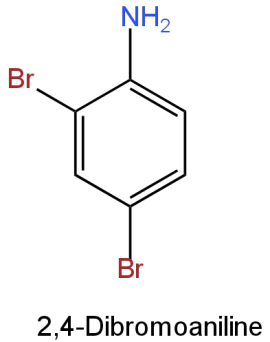
B.
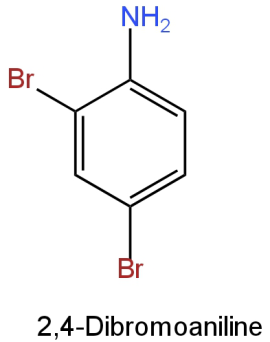
Image: Option B
This is 2,4-dibromoaniline (\[{{\rm{C}}_{\rm{6}}}{{\rm{H}}_{\rm{5}}}{\rm{B}}{{\rm{r}}_{\rm{2}}}{\rm{N}}\])
Molar mass \[{\rm{ = [12(6) + 5(1) + 2(80) + 14]g}}\] \[{\rm{ = 251g}}\]
It contains 2 bromine atoms.
So, the percentage of bromine
\[\begin{array}{*{20}{l}}{ = {\rm{ }}\left[ {\dfrac{{2\left( {80} \right)}}{{251}}} \right] \times 100}\\{ = {\rm{ }}63.74\% }\end{array}\]
This doesn't resemble the percentage of bromine in the given organic compound.
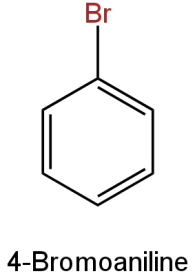
C.
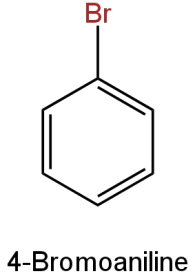
Image: Option C
This is 4-bromoaniline(\[{{\rm{C}}_{\rm{6}}}{{\rm{H}}_{\rm{6}}}{\rm{BrN}}\]).
Molar mass
\[{\rm{ = }}\left[ {{\rm{12}}\left( {\rm{6}} \right){\rm{ + 6}}\left( {\rm{1}} \right){\rm{ + }}\left( {{\rm{80}}} \right){\rm{ + 14}}} \right]{\rm{g}}\]
\[{\rm{ = 172g}}\]
It contains one bromine atom.
Percentage of bromine
\[ = \left( {\dfrac{{80}}{{172}}} \right) \times 100\]
\[ = 46.51\% \]
This is the same percentage as the bromine in the given organic compound.
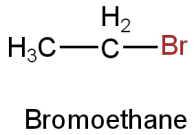
D.
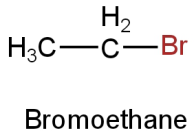
Image: Option D
This is bromoethane (\[{{\rm{C}}_{\rm{2}}}{{\rm{H}}_{\rm{5}}}{\rm{Br}}\]).
Molar mass \[{\rm{ = }}\left[ {{\rm{2}}\left( {{\rm{12}}} \right){\rm{ + 5}}\left( {\rm{1}} \right){\rm{ + 80}}} \right]{\rm{g}}\] \[{\rm{ = 109g}}\]
Only one bromine atom is present.
So, the percentage of bromine
\[ = {\rm{ }}\left[ {\left( {\dfrac{{80}}{{109}}} \right) \times 100} \right]\] \[ = 73.39\% \]
This doesn't resemble the percentage of bromine in the given organic compound.
So, option C is correct.
Additional Information:In the Carius method, a known mass of an organic compound is heated with fuming nitric acid in the presence of silver nitrate contained in a hard glass tube known as the Carius tube. Carbon and hydrogen that are present in the compound are oxidised to carbon dioxide and water.
The halogen present forms the corresponding silver halide.
It is filtered, washed, dried and weighed.
Note: While attending to the question, one must calculate the percentage of bromine present in each given option. He/she should have calculated the percentage of bromine in the given organic compound first. Then, after matching the bromine percentage in the given organic compound with the bromine percentage of individual options, the correct organic compound will be determined.
In this method, a halogen-containing organic compound is heated in the presence of silver nitrate.
Formula Used:
Percentage of halogen =
\[\dfrac{{{\rm{the atomic mass of bromine}}}}{{{\rm{mass of the organic compound}}}}{\rm{ }}\].×100
Complete Step by Step Solution:
The mass of the organic compound = 0.172g
Mass of bromine present in the organic compound = 0.08g
So, the percentage of bromine =\[\dfrac{{0.08}}{{0.172}}(100)\] \[ = 46.51\% \].
The atomic mass of bromine = 80g.
A.
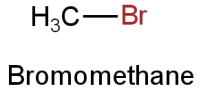
Image: Option A
This is bromomethane.
Molar mass
\[{\rm{ = [12 + 3(1) + 80]g}}\]
\[{\rm{ = 95g}}\]
The molar mass of bromoethane is 95g.
There is one bromine atom.
So, it contains 80g of bromine.
So, the percentage of bromine
\[ = \dfrac{{80}}{{95}} \times 100 = 84.21\% \]
This doesn't resemble the percentage of bromine in the given organic compound.
B.

Image: Option B
This is 2,4-dibromoaniline (\[{{\rm{C}}_{\rm{6}}}{{\rm{H}}_{\rm{5}}}{\rm{B}}{{\rm{r}}_{\rm{2}}}{\rm{N}}\])
Molar mass \[{\rm{ = [12(6) + 5(1) + 2(80) + 14]g}}\] \[{\rm{ = 251g}}\]
It contains 2 bromine atoms.
So, the percentage of bromine
\[\begin{array}{*{20}{l}}{ = {\rm{ }}\left[ {\dfrac{{2\left( {80} \right)}}{{251}}} \right] \times 100}\\{ = {\rm{ }}63.74\% }\end{array}\]
This doesn't resemble the percentage of bromine in the given organic compound.
C.

Image: Option C
This is 4-bromoaniline(\[{{\rm{C}}_{\rm{6}}}{{\rm{H}}_{\rm{6}}}{\rm{BrN}}\]).
Molar mass
\[{\rm{ = }}\left[ {{\rm{12}}\left( {\rm{6}} \right){\rm{ + 6}}\left( {\rm{1}} \right){\rm{ + }}\left( {{\rm{80}}} \right){\rm{ + 14}}} \right]{\rm{g}}\]
\[{\rm{ = 172g}}\]
It contains one bromine atom.
Percentage of bromine
\[ = \left( {\dfrac{{80}}{{172}}} \right) \times 100\]
\[ = 46.51\% \]
This is the same percentage as the bromine in the given organic compound.
D.

Image: Option D
This is bromoethane (\[{{\rm{C}}_{\rm{2}}}{{\rm{H}}_{\rm{5}}}{\rm{Br}}\]).
Molar mass \[{\rm{ = }}\left[ {{\rm{2}}\left( {{\rm{12}}} \right){\rm{ + 5}}\left( {\rm{1}} \right){\rm{ + 80}}} \right]{\rm{g}}\] \[{\rm{ = 109g}}\]
Only one bromine atom is present.
So, the percentage of bromine
\[ = {\rm{ }}\left[ {\left( {\dfrac{{80}}{{109}}} \right) \times 100} \right]\] \[ = 73.39\% \]
This doesn't resemble the percentage of bromine in the given organic compound.
So, option C is correct.
Additional Information:In the Carius method, a known mass of an organic compound is heated with fuming nitric acid in the presence of silver nitrate contained in a hard glass tube known as the Carius tube. Carbon and hydrogen that are present in the compound are oxidised to carbon dioxide and water.
The halogen present forms the corresponding silver halide.
It is filtered, washed, dried and weighed.
Note: While attending to the question, one must calculate the percentage of bromine present in each given option. He/she should have calculated the percentage of bromine in the given organic compound first. Then, after matching the bromine percentage in the given organic compound with the bromine percentage of individual options, the correct organic compound will be determined.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




