
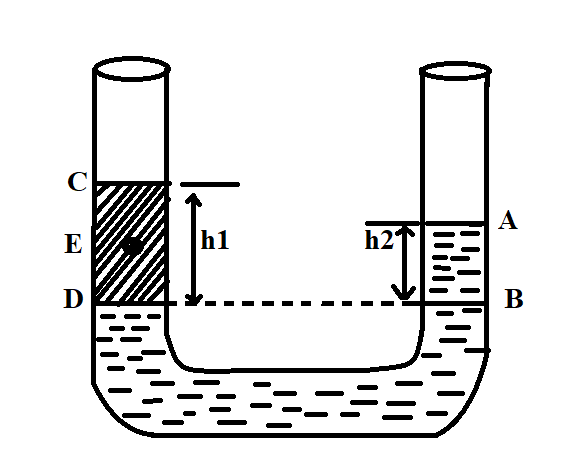
In a U-tube experiment, a column AB of water is balanced by a column CD of paraffin. The relative density of paraffin is:
A) $\dfrac{{{h_2}}}{{{h_1}}}$
B) $\dfrac{{{h_1}}}{{{h_2}}}$
C) $\dfrac{{{h_2} - {h_1}}}{{{h_2}}}$
D) $\dfrac{{{h_2}}}{{{h_1} + {h_2}}}$
Answer
240.3k+ views
Hint: Using Pascal’s law we get that, at the same horizontal level both fluids have the same pressure, i.e. pressure at B and pressure at D will be the same. In a confined incompressible liquid, the changes in pressure will be transmitted throughout the fluid. The pressure is directly proportional to the height and density of the fluid.
Formula used:
$P = {P_0} + h\rho g$ (where $P$ is the pressure due to the liquid, $h$ stands for the height of the liquid, $\rho $stands for the density of the liquid, and $g$ is the acceleration due to gravity)
Complete step by step solution:
According to Pascal’s law, the pressure due to two liquids at the same level will be equal. Thus we can equate the pressure due to both liquids on either side.
On the left side, the pressure is exerted by paraffin and on the right side, the pressure is exerted by water. By using Pascal’s law we can equate both.
$\therefore $Pressure at B= pressure at D
The total pressure at a point is given by the formula,
$P = {P_0} + h\rho g$…………………….
For Paraffin, the height and the density can be written as,
$h = {h_1} $
$ \rho = {\rho _p} $
For water, the height and the density can be written as,
$h = {h_2} $
$\rho = {\rho _w} $
Substituting the above values in equation (1)
${P_0} + {h_1}{\rho _p}g = {P_0} + {h_2}{\rho _w}g$
${h_1}{\rho _p} = {h_2}{\rho _w}$
Taking the ratio of densities, we get
$\dfrac{{{\rho _p}}}{{{\rho _w}}} = \dfrac{{{h_2}}}{{{h_1}}}$
Thus we can write the relative density as,
${\rho _r} = \dfrac{{{h_2}}}{{{h_1}}}$
The correct answer is option (A), $\dfrac{{{h_2}}}{{{h_1}}}.$
Note: The pressure is always acting normal to the area whatever maybe the orientation of the area. The pressure at a depth in a liquid is greater than the atmospheric pressure by an amount$h\rho g$, if the liquid is open to an atmosphere. The excess pressure at a depth h is called the gauge pressure at that point.
Formula used:
$P = {P_0} + h\rho g$ (where $P$ is the pressure due to the liquid, $h$ stands for the height of the liquid, $\rho $stands for the density of the liquid, and $g$ is the acceleration due to gravity)
Complete step by step solution:
According to Pascal’s law, the pressure due to two liquids at the same level will be equal. Thus we can equate the pressure due to both liquids on either side.
On the left side, the pressure is exerted by paraffin and on the right side, the pressure is exerted by water. By using Pascal’s law we can equate both.
$\therefore $Pressure at B= pressure at D
The total pressure at a point is given by the formula,
$P = {P_0} + h\rho g$…………………….
For Paraffin, the height and the density can be written as,
$h = {h_1} $
$ \rho = {\rho _p} $
For water, the height and the density can be written as,
$h = {h_2} $
$\rho = {\rho _w} $
Substituting the above values in equation (1)
${P_0} + {h_1}{\rho _p}g = {P_0} + {h_2}{\rho _w}g$
${h_1}{\rho _p} = {h_2}{\rho _w}$
Taking the ratio of densities, we get
$\dfrac{{{\rho _p}}}{{{\rho _w}}} = \dfrac{{{h_2}}}{{{h_1}}}$
Thus we can write the relative density as,
${\rho _r} = \dfrac{{{h_2}}}{{{h_1}}}$
The correct answer is option (A), $\dfrac{{{h_2}}}{{{h_1}}}.$
Note: The pressure is always acting normal to the area whatever maybe the orientation of the area. The pressure at a depth in a liquid is greater than the atmospheric pressure by an amount$h\rho g$, if the liquid is open to an atmosphere. The excess pressure at a depth h is called the gauge pressure at that point.
Recently Updated Pages
Dimensions of Charge: Dimensional Formula, Derivation, SI Units & Examples

How to Calculate Moment of Inertia: Step-by-Step Guide & Formulas

Circuit Switching vs Packet Switching: Key Differences Explained

Dimensions of Pressure in Physics: Formula, Derivation & SI Unit

JEE Extractive Metallurgy Important Concepts and Tips for Exam Preparation

JEE General Topics in Chemistry Important Concepts and Tips

Trending doubts
JEE Main 2026: Session 1 Results Out and Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

Ideal and Non-Ideal Solutions Explained for Class 12 Chemistry

JEE Main Participating Colleges 2026 - A Complete List of Top Colleges

Clemmensen and Wolff Kishner Reductions Explained for JEE & NEET

Degree of Dissociation: Meaning, Formula, Calculation & Uses

Understanding the Angle of Deviation in a Prism

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

JEE Advanced 2026 - Exam Date (Released), Syllabus, Registration, Eligibility, Preparation, and More

CBSE Notes Class 11 Physics Chapter 4 - Laws of Motion - 2025-26

CBSE Notes Class 11 Physics Chapter 14 - Waves - 2025-26

CBSE Notes Class 11 Physics Chapter 9 - Mechanical Properties of Fluids - 2025-26

Inductive Effect and Its Role in Acidic Strength




