
In a reaction
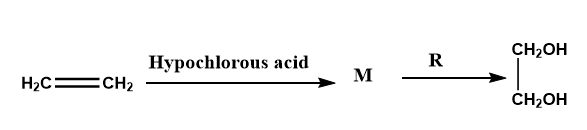
Where, M = Molecules, R = Reagent.
M and R are
(a) \[C{H_3}C{H_2}Cl\] and \[NaOH\]
(b) \[C{H_2}Cl - C{H_2}OH\] and aq. \[NaHC{O_3}\]
(c) \[C{H_3}C{H_2}OH\] and \[HCl\]
(d) \[C{H_2} = C{H_2}\] and heat
Answer
233.1k+ views
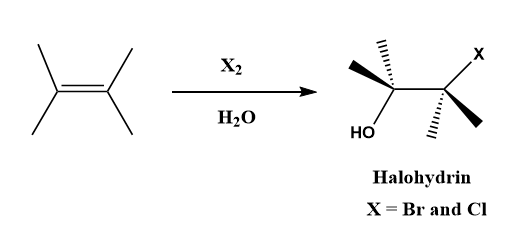
Hint: The halohydrin functional group containing a halogen and a hydroxyl group, which are connected to the adjacent carbon atoms. For example: 2-chloroethanol, 3-chloropropane-1,2-diol, etc. Bromohydrin and chlorohydrin are also examples of halohydrin.
Complete Step by Step Answer:
The addition of hypohalous acids (\[HOX\], \[X = Br,Cl\]) to the alkene is an example of an additional reaction. In the given question, we have to determine the molecule (M) and Reagent (R).
As we know that when a molecule of hypohalous acids (\[HOX\], \[X = Br,Cl\]) is added to the double bond of an alkene, the formation of \[C{H_2}Cl - C{H_2}OH\] i.e., formation of M is occurred.
\[C{H_2} = C{H_2} + HOCl \to C{H_2}Cl - C{H_2}OH\]
After that molecule, M undergoes hydrolysis with aqueous sodium bicarbonate (\[NaHC{O_3}\]) OR with reagent R to hydrolyze the \[C - Cl\] bond and form the ethylene glycol (\[C{H_2}OH - C{H_2}OH\]).
\[\begin{array}{l}C{H_2}Cl - C{H_2}OH + aq.NaHC{O_3} \to C{H_2}OH - C{H_2}OH\\\end{array}\]
Like the electrophilic addition reaction of an alkene with bromine, the formation of halohydrin also involves the electrophilic addition reaction, in which anti-addition of chlorine and hydroxyl functional groups occurs with the formation of trans products.
In the question, options (a), (c), and (d) will be the incorrect option because the structure of M is \[C{H_2}Cl - C{H_2}OH\]and reagent R is \[NaHC{O_3}\].
Therefore, we can conclude that option (b) will be the correct answer.
Note: The halohydrins are not formed by the direct addition of hypohalous acid (\[HOX\]), instead the alkene reacts with bromine (\[B{r_2}\]) or chlorine (\[C{l_2}\]) in the presence of water(\[{H_2}O\]). Sodium bicarbonate (\[NaHC{O_3}\]) is also known as baking soda.

Complete Step by Step Answer:
The addition of hypohalous acids (\[HOX\], \[X = Br,Cl\]) to the alkene is an example of an additional reaction. In the given question, we have to determine the molecule (M) and Reagent (R).
As we know that when a molecule of hypohalous acids (\[HOX\], \[X = Br,Cl\]) is added to the double bond of an alkene, the formation of \[C{H_2}Cl - C{H_2}OH\] i.e., formation of M is occurred.
\[C{H_2} = C{H_2} + HOCl \to C{H_2}Cl - C{H_2}OH\]
After that molecule, M undergoes hydrolysis with aqueous sodium bicarbonate (\[NaHC{O_3}\]) OR with reagent R to hydrolyze the \[C - Cl\] bond and form the ethylene glycol (\[C{H_2}OH - C{H_2}OH\]).
\[\begin{array}{l}C{H_2}Cl - C{H_2}OH + aq.NaHC{O_3} \to C{H_2}OH - C{H_2}OH\\\end{array}\]
Like the electrophilic addition reaction of an alkene with bromine, the formation of halohydrin also involves the electrophilic addition reaction, in which anti-addition of chlorine and hydroxyl functional groups occurs with the formation of trans products.
In the question, options (a), (c), and (d) will be the incorrect option because the structure of M is \[C{H_2}Cl - C{H_2}OH\]and reagent R is \[NaHC{O_3}\].
Therefore, we can conclude that option (b) will be the correct answer.
Note: The halohydrins are not formed by the direct addition of hypohalous acid (\[HOX\]), instead the alkene reacts with bromine (\[B{r_2}\]) or chlorine (\[C{l_2}\]) in the presence of water(\[{H_2}O\]). Sodium bicarbonate (\[NaHC{O_3}\]) is also known as baking soda.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




