
${H_3}B{O_3}$ is a:
(A) Monobasic and Lewis acid
(B) Monobasic and Bronsted acid
(C) Tribasic and Lewis acid
(D) Tribasic and Lewis acid
Answer
233.1k+ views
Hint: Basicity of an acid depends upon the number of protons it can give upon its dissolution in an aqueous medium. Boric acid contains three –OH groups in its structure.
Complete step by step solution:
Let’s see which type of acid ${H_3}B{O_3}$ (Boric acid) is.
-Basicity of the acids is the number of protons an acid can give upon dissociation in an aqueous solution. So, if an acid can give one proton upon dissociation in aqueous solution, then the acid is said to be monoprotic. Thus, a diprotic acid will give two protons upon dissociation in aqueous solution.
-The chemical structure of boric acid and its reaction in an aqueous medium can be given as
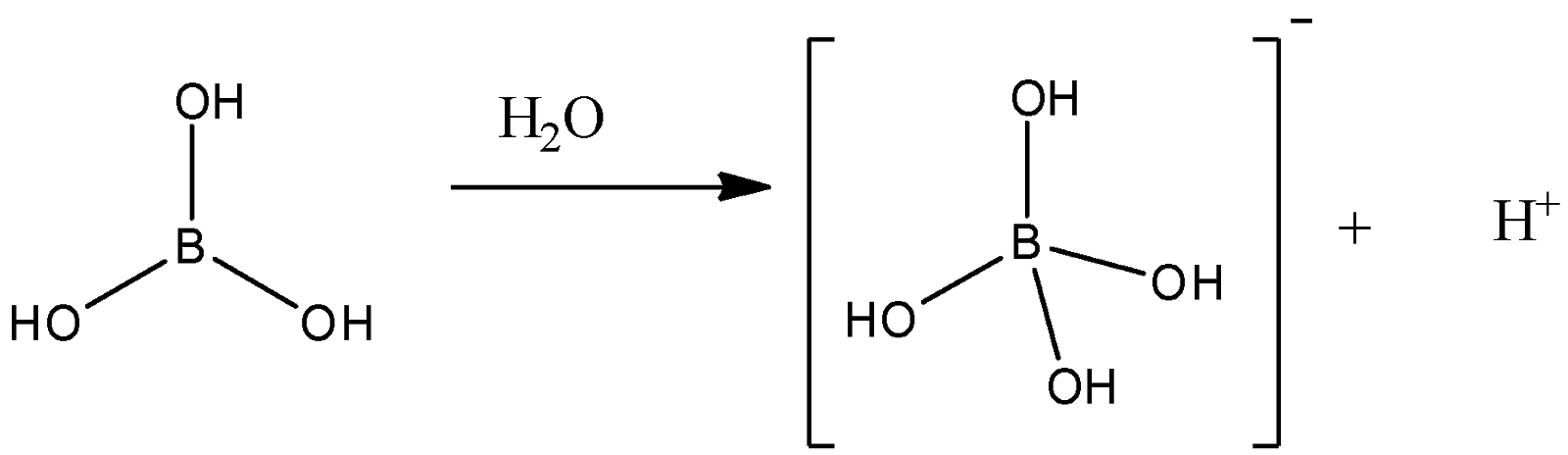
-Here, we can say that though it has three protons, it will not act as a proton donor. It will react with water molecules to form ${[B{(OH)_4}]^ - }$ and will give one proton. So, in this way, it will act as an acid.
-Here, we can see that one molecule of boric acid gives one proton. So, this acid can be considered as a monoprotic acid.
-Bronsted acid is an acid which is capable of donating a proton from its molecule. Boric acid is not able to donate the proton. So, it is not a Bronsted acid.
-Lewis acid is an acid that can accept electron pairs from the base. Here Boric acid can accept the electron pair from water molecules as shown in the reaction. Thus, Boric acid is a Lewis acid.
So, we can conclude that Boric acid is a monoprotic and Lewis acid.
Therefore, the correct answer is (A).
Note: The most common mistake we make here is that we consider Boric acid as a triprotic acid. Actually, it is a Lewis acid also and so that it will form a complex ${[B{(OH)_4}]^ - }$ with water. As this complex is formed, it is not able to donate three protons and actually, it is able to donate only one proton.
Complete step by step solution:
Let’s see which type of acid ${H_3}B{O_3}$ (Boric acid) is.
-Basicity of the acids is the number of protons an acid can give upon dissociation in an aqueous solution. So, if an acid can give one proton upon dissociation in aqueous solution, then the acid is said to be monoprotic. Thus, a diprotic acid will give two protons upon dissociation in aqueous solution.
-The chemical structure of boric acid and its reaction in an aqueous medium can be given as

-Here, we can say that though it has three protons, it will not act as a proton donor. It will react with water molecules to form ${[B{(OH)_4}]^ - }$ and will give one proton. So, in this way, it will act as an acid.
-Here, we can see that one molecule of boric acid gives one proton. So, this acid can be considered as a monoprotic acid.
-Bronsted acid is an acid which is capable of donating a proton from its molecule. Boric acid is not able to donate the proton. So, it is not a Bronsted acid.
-Lewis acid is an acid that can accept electron pairs from the base. Here Boric acid can accept the electron pair from water molecules as shown in the reaction. Thus, Boric acid is a Lewis acid.
So, we can conclude that Boric acid is a monoprotic and Lewis acid.
Therefore, the correct answer is (A).
Note: The most common mistake we make here is that we consider Boric acid as a triprotic acid. Actually, it is a Lewis acid also and so that it will form a complex ${[B{(OH)_4}]^ - }$ with water. As this complex is formed, it is not able to donate three protons and actually, it is able to donate only one proton.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




