
Give the IUPAC names of the following ethers:
Given ether:
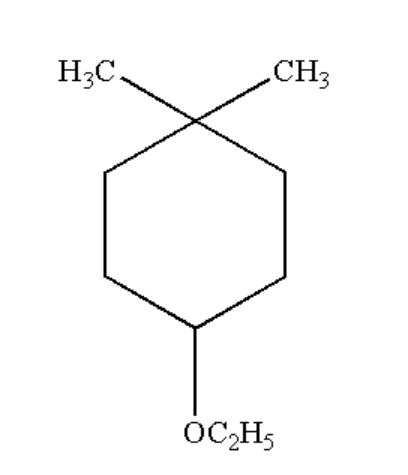
Answer
233.1k+ views
Hint: First determine the parent chain containing a maximum number of carbon atoms. Cycloalkanes are cyclic compounds where no double bonding or triple bonding is present. In IUPAC naming the term is used as a suffix.
Complete step by step solution:
Ether functional groups consisting of chemical compounds are determined by the general formula $R - O - R'$ , where R is the alkyl group.
International Union of Pure and Applied Chemistry are the sets of rules that are applied to determine the chemical name of the compound. This is known as the IUPAC naming system.
The given chemical compound is shown below.
Numbering of Carbon atom
2. Determination of names and position of locants: In the given compound, one “Ethoxy” group is attached to the 4th position and two “Methyl” groups are attached to the first position.
3. Determination of suffix: As there is no availability of double or triple bonds, it is considered a saturated hydrocarbon. The suffix used will be “ane”.
The IUPAC name will be 4-ethoxy-1,1-dimethylcyclohexane.
Hence, the answer is “4-ethoxy-1,1-dimethylcyclohexane”.
Note: The group with less number of carbon atoms attached to the oxygen is treated as a substituent group whereas the group comprising more number of carbon atoms is considered as the parent group. Always the substituent group is written prior to the parent group. As the ethyl group is attached to the oxygen atom, therefore the substituent group is termed “Ethoxy”.
Complete step by step solution:
Ether functional groups consisting of chemical compounds are determined by the general formula $R - O - R'$ , where R is the alkyl group.
International Union of Pure and Applied Chemistry are the sets of rules that are applied to determine the chemical name of the compound. This is known as the IUPAC naming system.
The given chemical compound is shown below.
Numbering of Carbon atom
- 1. Determination of Root Word: In the given compound, the maximum number of carbon atoms present in a continuous chain is 6, so the hydrocarbon is hexane and the root word is “Hex”. As it is a cyclic compound then the hydrocarbon will be referred to as cyclohexane.
The IUPAC name will be 4-ethoxy-1,1-dimethylcyclohexane.
Hence, the answer is “4-ethoxy-1,1-dimethylcyclohexane”.
Note: The group with less number of carbon atoms attached to the oxygen is treated as a substituent group whereas the group comprising more number of carbon atoms is considered as the parent group. Always the substituent group is written prior to the parent group. As the ethyl group is attached to the oxygen atom, therefore the substituent group is termed “Ethoxy”.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




