
Find the number of $P-O$ bonds of identical length in hypophosphate ion.
Answer
233.1k+ views
Hint: Draw the structure of hypophosphate ion and then count the number of $P-O$ bonds in it which are of identical length.
Complete Step by Step Answer:
The oxyanion of Hypophosphoric acid ${{H}_{2}}P{{O}_{3}}$ ${{H}_{4}}{{P}_{2}}{{O}_{6}}$ is hypophosphate ion(${{P}_{2}}{{O}_{6}}^{4-}$). The structure of this ion has been a subject of speculation and controversy since hypophosphoric acid was discovered by Salzer in 1877 . Bell and Sugden showed conclusively that the salts which they studied were not derived from an acid of simple structure like ${{H}_{2}}P{{O}_{3}}$ and of the possible dimeric formulae for the acid . In this way the structure for this hypophosphate ion is represented finally as:
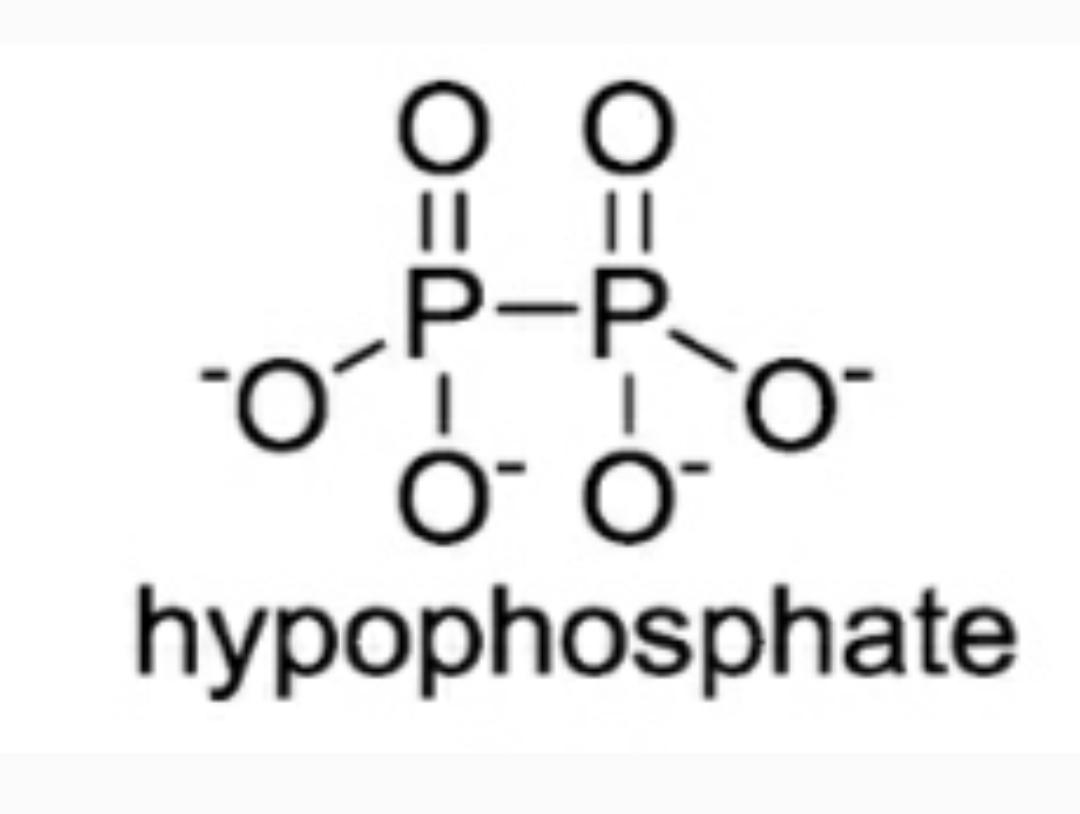
Now since here in this structure there are negative charges present and double bonds on oxygen atoms, all atomic bonds between P and O( $P-O$) do resonate. As we know that in resonance all the bond lengths are of identical length due to the regular shift of double bond in each bond respectively. Thus , if we count the number of these bonds then we get the total number as 6.
Thus , the correct answer is 6.
Note: It should be known that when negative charge is present on an electronegative ion like that on oxygen and double bonds are present then resonance occurs and due to resonance only the total number of bonds will be 6. Some students take into account only the single bonds of $P-O$ and declare the final answer as 4 which is the wrong answer.
Complete Step by Step Answer:
The oxyanion of Hypophosphoric acid ${{H}_{2}}P{{O}_{3}}$ ${{H}_{4}}{{P}_{2}}{{O}_{6}}$ is hypophosphate ion(${{P}_{2}}{{O}_{6}}^{4-}$). The structure of this ion has been a subject of speculation and controversy since hypophosphoric acid was discovered by Salzer in 1877 . Bell and Sugden showed conclusively that the salts which they studied were not derived from an acid of simple structure like ${{H}_{2}}P{{O}_{3}}$ and of the possible dimeric formulae for the acid . In this way the structure for this hypophosphate ion is represented finally as:

Now since here in this structure there are negative charges present and double bonds on oxygen atoms, all atomic bonds between P and O( $P-O$) do resonate. As we know that in resonance all the bond lengths are of identical length due to the regular shift of double bond in each bond respectively. Thus , if we count the number of these bonds then we get the total number as 6.
Thus , the correct answer is 6.
Note: It should be known that when negative charge is present on an electronegative ion like that on oxygen and double bonds are present then resonance occurs and due to resonance only the total number of bonds will be 6. Some students take into account only the single bonds of $P-O$ and declare the final answer as 4 which is the wrong answer.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




