
Ethylene difluoride on hydrolysis gives
A. Glycol
B. Fluoroethanol
C. Difluoroethanol
D. Freon
Answer
233.4k+ views
Hint: Hydrolysis is a chemical interaction in which a water molecule breaks down one or more chemical bonds. Water acts as a nucleophile in this reaction.
Complete Step by Step Solution:
Ethylene difluoride or 1,2-difluoro ethane is a fluorinated hydrocarbon.
In the hydrolysis of ethylene difluoride, the carbon-fluorine bond is broken down.
A. Glycol
It is also known as ethylene glycol.
The chemical formula is \[{\left( {{\rm{C}}{{\rm{H}}_{\rm{2}}}{\rm{OH}}} \right)_{\rm{2}}}\].
When ethylene difluoride is treated with water, the hydroxide ions act as a nucleophile.
These hydroxide ions attack the reactant and replace the fluoride ions.
Two hydroxide ions replace two fluoride ions.
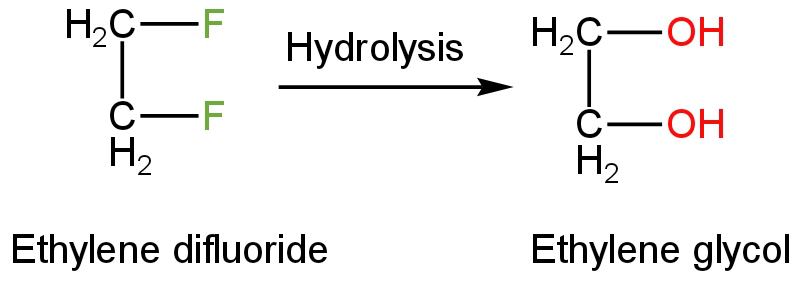
Image: Hydrolysis of ethylene difluoride
The product formed is a liquid having no odour or colour, sweet taste, explosive, dense liquid.
So, A is correct.
B. Fluoroethanol
2-Fluoroethanol is an organic compound with the chemical formula \[{\rm{C}}{{\rm{H}}_2}{\rm{FC}}{{\rm{H}}_2}{\rm{OH}}\].
This is a liquid with no colour and is one of the simple stable fluorinated alcohols.
Its structure is as follows:
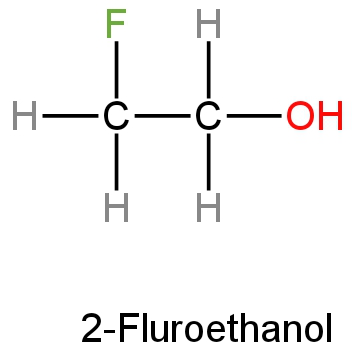
Image: 2-Fluoroethanol
In the hydrolysis of ethylene difluoride, both the fluorine atoms are replaced by hydroxide ions.
So, this is not the product of the hydrolysis of ethylene difluoride.
So, B is incorrect.
C. Difluoroethanol
Difluoroethanol is an organic compound with the chemical formula \[{\rm{C}}{{\rm{F}}_2}{\rm{HC}}{{\rm{H}}_2}{\rm{OH}}\].
This is a liquid with no colour and is one of the simple stable fluorinated alcohols.
Its structure is as follows:
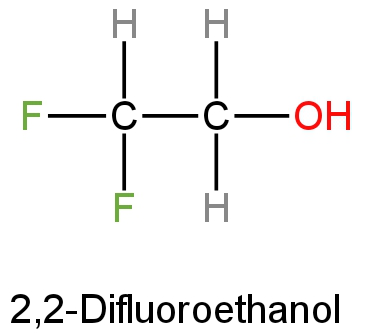
Image: Difluoroethanol
In the hydrolysis of ethylene difluoride, both the fluorine atoms are replaced by hydroxide ions.
So, this is not the product of the hydrolysis of ethylene difluoride.
So, C is incorrect.
D. Freon
Chlorofluoro compounds of methane and ethane in which all the hydrogen atoms are replaced by halogen atoms are altogether known as freons.
There are different types of freons depending on the number of fluorine atoms present in them.
For example,
\[{\rm{C}}{{\rm{F}}_{\rm{4}}}\] or Freon-14,
\[{\rm{C}}{{\rm{F}}_{\rm{2}}}{\rm{C}}{{\rm{l}}_{\rm{2}}}\] or Freon-12, \[{\rm{CFC}}{{\rm{l}}_{\rm{3}}}\] or Freon-11.
In the hydrolysis of ethylene difluoride, both the fluorine atoms are replaced by hydroxide ions.
So, this is not the product of hydrolysis of ethylene difluoride.
So, D is incorrect.
So, option A is correct.
Note: While attending to the question, one must remember that the hydrolysis of ethylene difluoride involves the breaking of the carbon-fluorine bond. New carbon-oxygen bonds are formed. A rough idea about the chemical structure of all the given options must be there.
Complete Step by Step Solution:
Ethylene difluoride or 1,2-difluoro ethane is a fluorinated hydrocarbon.
In the hydrolysis of ethylene difluoride, the carbon-fluorine bond is broken down.
A. Glycol
It is also known as ethylene glycol.
The chemical formula is \[{\left( {{\rm{C}}{{\rm{H}}_{\rm{2}}}{\rm{OH}}} \right)_{\rm{2}}}\].
When ethylene difluoride is treated with water, the hydroxide ions act as a nucleophile.
These hydroxide ions attack the reactant and replace the fluoride ions.
Two hydroxide ions replace two fluoride ions.

Image: Hydrolysis of ethylene difluoride
The product formed is a liquid having no odour or colour, sweet taste, explosive, dense liquid.
So, A is correct.
B. Fluoroethanol
2-Fluoroethanol is an organic compound with the chemical formula \[{\rm{C}}{{\rm{H}}_2}{\rm{FC}}{{\rm{H}}_2}{\rm{OH}}\].
This is a liquid with no colour and is one of the simple stable fluorinated alcohols.
Its structure is as follows:

Image: 2-Fluoroethanol
In the hydrolysis of ethylene difluoride, both the fluorine atoms are replaced by hydroxide ions.
So, this is not the product of the hydrolysis of ethylene difluoride.
So, B is incorrect.
C. Difluoroethanol
Difluoroethanol is an organic compound with the chemical formula \[{\rm{C}}{{\rm{F}}_2}{\rm{HC}}{{\rm{H}}_2}{\rm{OH}}\].
This is a liquid with no colour and is one of the simple stable fluorinated alcohols.
Its structure is as follows:

Image: Difluoroethanol
In the hydrolysis of ethylene difluoride, both the fluorine atoms are replaced by hydroxide ions.
So, this is not the product of the hydrolysis of ethylene difluoride.
So, C is incorrect.
D. Freon
Chlorofluoro compounds of methane and ethane in which all the hydrogen atoms are replaced by halogen atoms are altogether known as freons.
There are different types of freons depending on the number of fluorine atoms present in them.
For example,
\[{\rm{C}}{{\rm{F}}_{\rm{4}}}\] or Freon-14,
\[{\rm{C}}{{\rm{F}}_{\rm{2}}}{\rm{C}}{{\rm{l}}_{\rm{2}}}\] or Freon-12, \[{\rm{CFC}}{{\rm{l}}_{\rm{3}}}\] or Freon-11.
In the hydrolysis of ethylene difluoride, both the fluorine atoms are replaced by hydroxide ions.
So, this is not the product of hydrolysis of ethylene difluoride.
So, D is incorrect.
So, option A is correct.
Note: While attending to the question, one must remember that the hydrolysis of ethylene difluoride involves the breaking of the carbon-fluorine bond. New carbon-oxygen bonds are formed. A rough idea about the chemical structure of all the given options must be there.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




