
$(E) - 3 - $bromo$ - 3 - $hexene when treated with $C{H_3}{O^ - }$ in $C{H_3}OH$ gives:
A. $3 - $hexyne
B. $2 - $hexyne
C. $2,3 - $ hexadiene
D. $2,4 - $hexadiene
Answer
233.1k+ views
Hint : We can get the product of the given reaction with the help of Saytzeff’s rule which is used in analysis of elimination reactions of organic chemistry. Elimination reaction of any halides and alcohol produces different alkenes. In such a type of reaction Saytzeff’s rule is used to predict major products. According to this rule, stable alkene is formed if the removal of hydrogen from $\beta - $ carbon which has a low number of substituents. This rule is applicable for dehydrohalogenation reactions. This problem is also based on dehydrobromination of the reactant so the product will be formed with the help of Saytzeff's rule.
Complete step by step solution:
So we can say that according to the Satzev’s rule $(E) - 3 - $bromo$ - 3 - $hexene when treated with $C{H_3}{O^ - }$ in $C{H_3}OH$ it produce $3 - $hexyne. It undergoes dehydrobromination where a molecule of hydrobromide gets eliminated from $(E) - 3 - $bromo$ - 3 - $hexene. A dehydrobromination reaction is a kind of elimination reaction where elimination of hydrogen bromide from a substrate takes place. This reaction is mainly associated with the synthesis of alkenes. The reaction of $(E) - 3 - $bromo$ - 3 - $hexene when treated with $C{H_3}{O^ - }$ in $C{H_3}OH$ is given below.
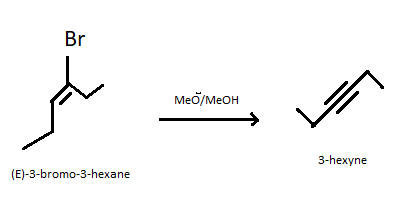
Hence option A is correct that is $(E) - 3 - $bromo$ - 3 - $hexene when treated with $C{H_3}{O^ - }$ in $C{H_3}OH$ gives $3 - $hexyne.
Note : We have approached this reaction with the help of saytzeff rule which favours the alkene with less number of hydrogen on double bonded carbon atoms.As the reaction is dehydrobromination so saytzeff rule is applicable here. We know that during the elimination reaction proton is removed from that carbon atom which have less number of substituents.Therefore $(E) - 3 - $bromo$ - 3 - $hexene when treated with $C{H_3}{O^ - }$ in $C{H_3}OH$ gives $3 - $hexyne.
Complete step by step solution:
So we can say that according to the Satzev’s rule $(E) - 3 - $bromo$ - 3 - $hexene when treated with $C{H_3}{O^ - }$ in $C{H_3}OH$ it produce $3 - $hexyne. It undergoes dehydrobromination where a molecule of hydrobromide gets eliminated from $(E) - 3 - $bromo$ - 3 - $hexene. A dehydrobromination reaction is a kind of elimination reaction where elimination of hydrogen bromide from a substrate takes place. This reaction is mainly associated with the synthesis of alkenes. The reaction of $(E) - 3 - $bromo$ - 3 - $hexene when treated with $C{H_3}{O^ - }$ in $C{H_3}OH$ is given below.

Hence option A is correct that is $(E) - 3 - $bromo$ - 3 - $hexene when treated with $C{H_3}{O^ - }$ in $C{H_3}OH$ gives $3 - $hexyne.
Note : We have approached this reaction with the help of saytzeff rule which favours the alkene with less number of hydrogen on double bonded carbon atoms.As the reaction is dehydrobromination so saytzeff rule is applicable here. We know that during the elimination reaction proton is removed from that carbon atom which have less number of substituents.Therefore $(E) - 3 - $bromo$ - 3 - $hexene when treated with $C{H_3}{O^ - }$ in $C{H_3}OH$ gives $3 - $hexyne.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




