
Dacron is an example of:
(a) Polyamides
(b) Polypropenes
(c) Polyacrylonitrile
(d) Polyesters
Answer
232.8k+ views
Hint: Dacron is an example of the family of polymers which is a petroleum-based synthetic fibre. These contain ester groups.
Complete step by step solution:
-Polyester is a category of polymers which contain ester functional groups. Dacron contains an ester functional group.
-Polyethylene terephthalate also known as Dacron is a most common thermoplastic polymer resin of polyester family of polymers. It’s commonly abbreviated as PET or PETE etc.
-IT is used in fibres for clothing, containers for liquids and foods. It is also used in combination with glass fibre for engineering resins.
-Its chemical representation is
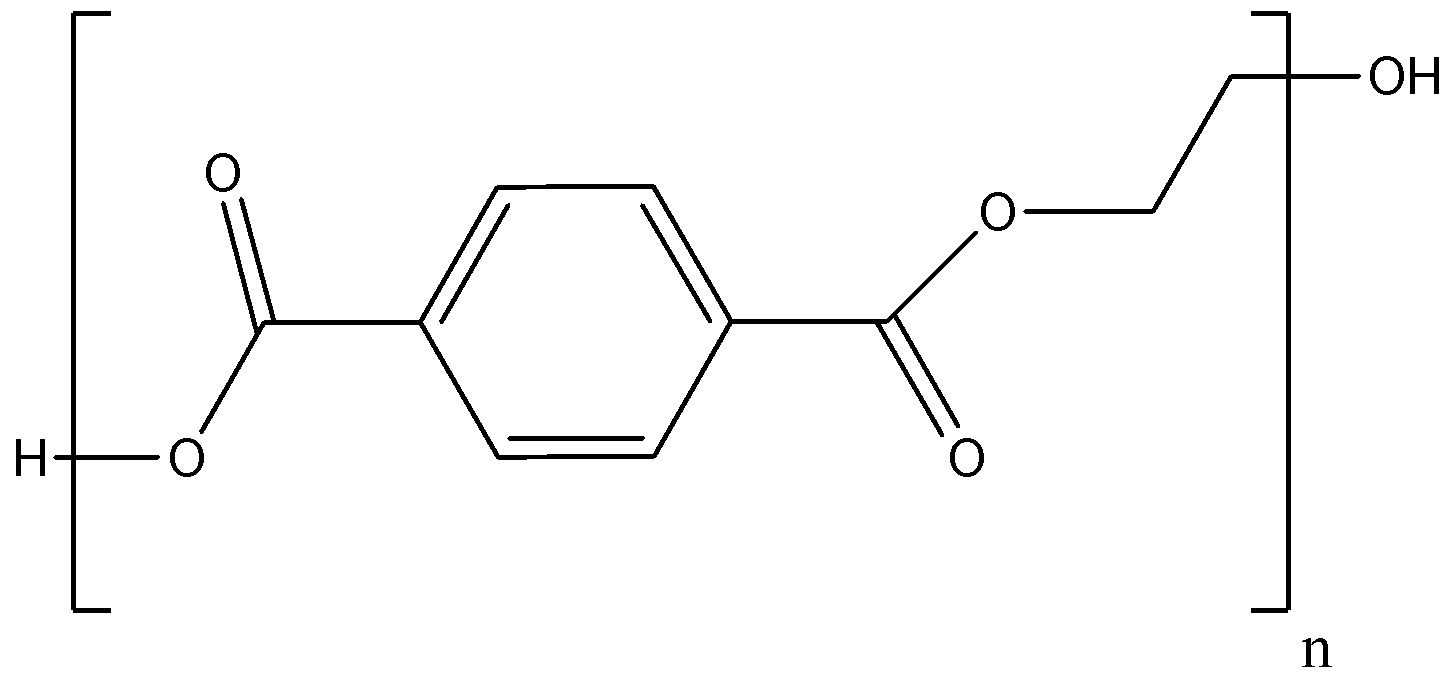
-Its monomer units are ethylene terephthalate. The monomer bis(2-hydroxyethyl) terephthalate is synthesized by the esterification reaction between terephthalic acid and ethylene glycol. It can also be synthesized by transesterification between ethylene glycol and dimethyl terephthalate(DMT) with methanol as a by-product.
-Dacron or PET in its natural state is colourless, semi-crystalline resin. When processed, it becomes semi-rigid and lightweight. It is very strong and is impact-resistant. When exposed to chloroform or other certain chemicals, PET turns white.
-Plastic bottles are made from PET which is used for soft drinks. When modified can be used for flexible food packaging and thermal insulation (such as space blankets). IT has a high mechanical strength and due to this it is also used in tape applications, such as magnetic tape or backing for pressure-sensitive adhesive tapes.
Thus, as Dacron consists of ester groups, it is a polyester and the option (d) is the correct answer to the question.
Note: There are many other names by which Darcon is known. It is known as Terylene in the United Kingdom and it is known as Lavsan in Russia. The name “Dacron” is mainly used in the United States.
Complete step by step solution:
-Polyester is a category of polymers which contain ester functional groups. Dacron contains an ester functional group.
-Polyethylene terephthalate also known as Dacron is a most common thermoplastic polymer resin of polyester family of polymers. It’s commonly abbreviated as PET or PETE etc.
-IT is used in fibres for clothing, containers for liquids and foods. It is also used in combination with glass fibre for engineering resins.
-Its chemical representation is

-Its monomer units are ethylene terephthalate. The monomer bis(2-hydroxyethyl) terephthalate is synthesized by the esterification reaction between terephthalic acid and ethylene glycol. It can also be synthesized by transesterification between ethylene glycol and dimethyl terephthalate(DMT) with methanol as a by-product.
-Dacron or PET in its natural state is colourless, semi-crystalline resin. When processed, it becomes semi-rigid and lightweight. It is very strong and is impact-resistant. When exposed to chloroform or other certain chemicals, PET turns white.
-Plastic bottles are made from PET which is used for soft drinks. When modified can be used for flexible food packaging and thermal insulation (such as space blankets). IT has a high mechanical strength and due to this it is also used in tape applications, such as magnetic tape or backing for pressure-sensitive adhesive tapes.
Thus, as Dacron consists of ester groups, it is a polyester and the option (d) is the correct answer to the question.
Note: There are many other names by which Darcon is known. It is known as Terylene in the United Kingdom and it is known as Lavsan in Russia. The name “Dacron” is mainly used in the United States.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




