
Common monomer present in Melamine Formaldehyde polymer and Bakelite is
(A) Formaldehyde
(B) Phenol
(C) Melamine
(D) Ethylene Glycol
Answer
240k+ views
Hint: It is an aldehydic simple chemical compound made of hydrogen, oxygen and carbon. It is a colourless compound which has a pungent smell.
Complete step by step solution:
Now, let us know about the formation of Melamine Formaldehyde
-Melamine (1, 3, 5-triamino-2, 4, 6-triazine) formaldehyde.
-Melamine formaldehyde (MF) polymers are primarily made up of melamine and formaldehyde with formaldehyde.
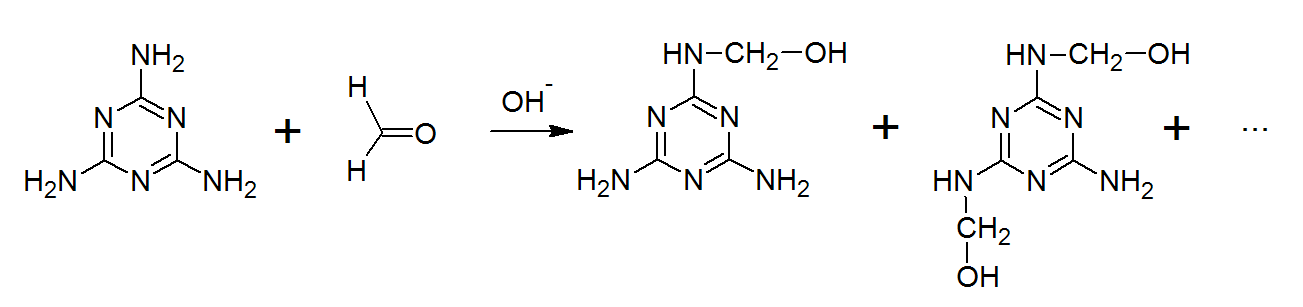
-Monomers of Melamine Formaldehyde are Melamine and Formaldehyde.
Additional Information:
-Melamine formaldehyde is a hard, durable, and versatile thermosetting amyloplast with good fire and heat resistance.
-It is an amino resin and has various material advantages, such as transparency, better hardness, thermal stability, excellent boil resistance, scratch resistance, abrasion resistance, flame retardant, moisture resistance and surface smoothness, which lead MF to large industrial applications.
Now, we learn the preparation of Bakelite
-Bakelite ${({C_6} - {H_6} - O.C - {H_2} - O)_x}$
-Bakelite is produced through condensation reaction between phenol and formaldehyde in the presence of either basic or acidic catalyst.
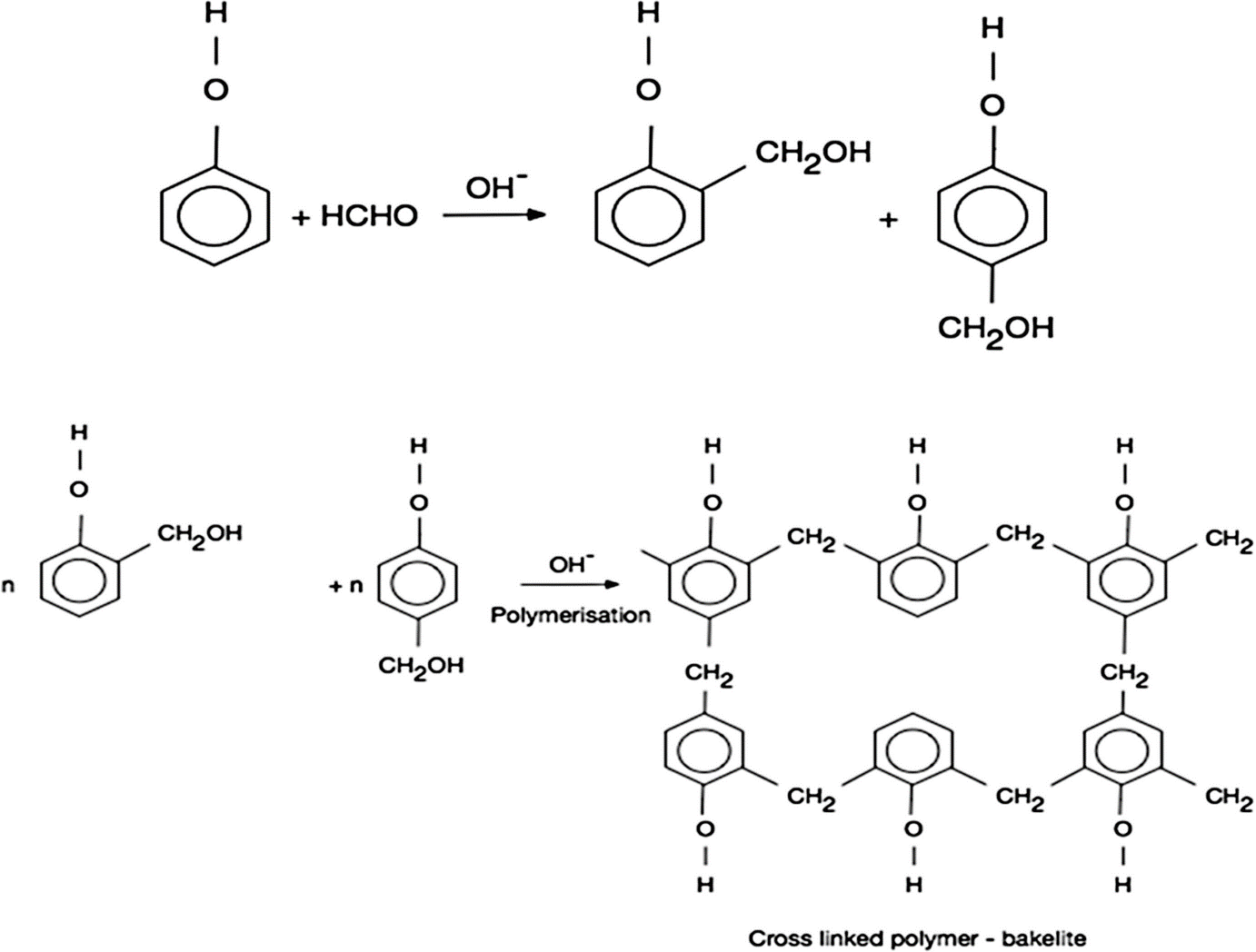
-Monomers of Bakelite are Phenol and Formaldehyde.
Hence, the common monomer of Melamine Formaldehyde and Bakelite is Formaldehyde. Therefore, Option (A)is correct.
Additional information:
-It is a thermo-setting and cross-linked polymer.
-Bakelite is a non-conductive and heat resistant material that makes it ideal for electrical insulators. It’s rigid, hard, scratch-resistant, infusible, water-resistant, insoluble solid and a good insulator.
-It was used for its non-conductive and heat-resistant properties in electrical insulators, and also in radios and telephones.
By learning the preparation of Bakelite and Melamine Formaldehyde we got to know that the monomers used for the preparation of Melamine are Melamine and Formaldehyde and the monomers used for the preparation of Bakelite are Formaldehyde and Phenol.
Note: 1. Phenol and Formaldehyde are required for the formation of Bakelite whereas Melamine and Formaldehyde are required for Melamine Formaldehyde polymer.
2. Do not get confused by the occurrence of Phenol and Melamine as the two are also monomers.
Complete step by step solution:
Now, let us know about the formation of Melamine Formaldehyde
-Melamine (1, 3, 5-triamino-2, 4, 6-triazine) formaldehyde.
-Melamine formaldehyde (MF) polymers are primarily made up of melamine and formaldehyde with formaldehyde.

-Monomers of Melamine Formaldehyde are Melamine and Formaldehyde.
Additional Information:
-Melamine formaldehyde is a hard, durable, and versatile thermosetting amyloplast with good fire and heat resistance.
-It is an amino resin and has various material advantages, such as transparency, better hardness, thermal stability, excellent boil resistance, scratch resistance, abrasion resistance, flame retardant, moisture resistance and surface smoothness, which lead MF to large industrial applications.
Now, we learn the preparation of Bakelite
-Bakelite ${({C_6} - {H_6} - O.C - {H_2} - O)_x}$
-Bakelite is produced through condensation reaction between phenol and formaldehyde in the presence of either basic or acidic catalyst.

-Monomers of Bakelite are Phenol and Formaldehyde.
Hence, the common monomer of Melamine Formaldehyde and Bakelite is Formaldehyde. Therefore, Option (A)is correct.
Additional information:
-It is a thermo-setting and cross-linked polymer.
-Bakelite is a non-conductive and heat resistant material that makes it ideal for electrical insulators. It’s rigid, hard, scratch-resistant, infusible, water-resistant, insoluble solid and a good insulator.
-It was used for its non-conductive and heat-resistant properties in electrical insulators, and also in radios and telephones.
By learning the preparation of Bakelite and Melamine Formaldehyde we got to know that the monomers used for the preparation of Melamine are Melamine and Formaldehyde and the monomers used for the preparation of Bakelite are Formaldehyde and Phenol.
Note: 1. Phenol and Formaldehyde are required for the formation of Bakelite whereas Melamine and Formaldehyde are required for Melamine Formaldehyde polymer.
2. Do not get confused by the occurrence of Phenol and Melamine as the two are also monomers.
Recently Updated Pages
Types of Solutions in Chemistry: Explained Simply

JEE Main Mock Test 2025-26: Principles Related To Practical

JEE Main 2025-26 Organic Compounds Containing Nitrogen Mock Test

JEE Main 2025-26 Mock Test: Organic Compounds Containing Oxygen

JEE Main 2025-26 Redox Reactions & Electro Mock Test

JEE Main Solutions Mock Test 1-2 (2025-26): Free Practice & Answers

Trending doubts
JEE Main 2026: Session 1 Results Out and Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

Ideal and Non-Ideal Solutions Explained for Class 12 Chemistry

JEE Main Participating Colleges 2026 - A Complete List of Top Colleges

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

JEE Advanced 2026 - Exam Date (Released), Syllabus, Registration, Eligibility, Preparation, and More

NCERT Solutions For Class 12 Chemistry Chapter 2 Electrochemistry - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The D And F Block Elements - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules - 2025-26




