




Isomerism in Organic Compounds: A Brief Introduction
Isomerism is the phenomenon of two or more organic compounds having the same molecular formula but one different physical or chemical property. It occurs due to the difference in the spatial arrangement of atoms (stereoisomers) or due to different carbon skeletons (structural isomerism). Structural isomers are further subdivided into six types, and stereoisomers are subdivided into two types.
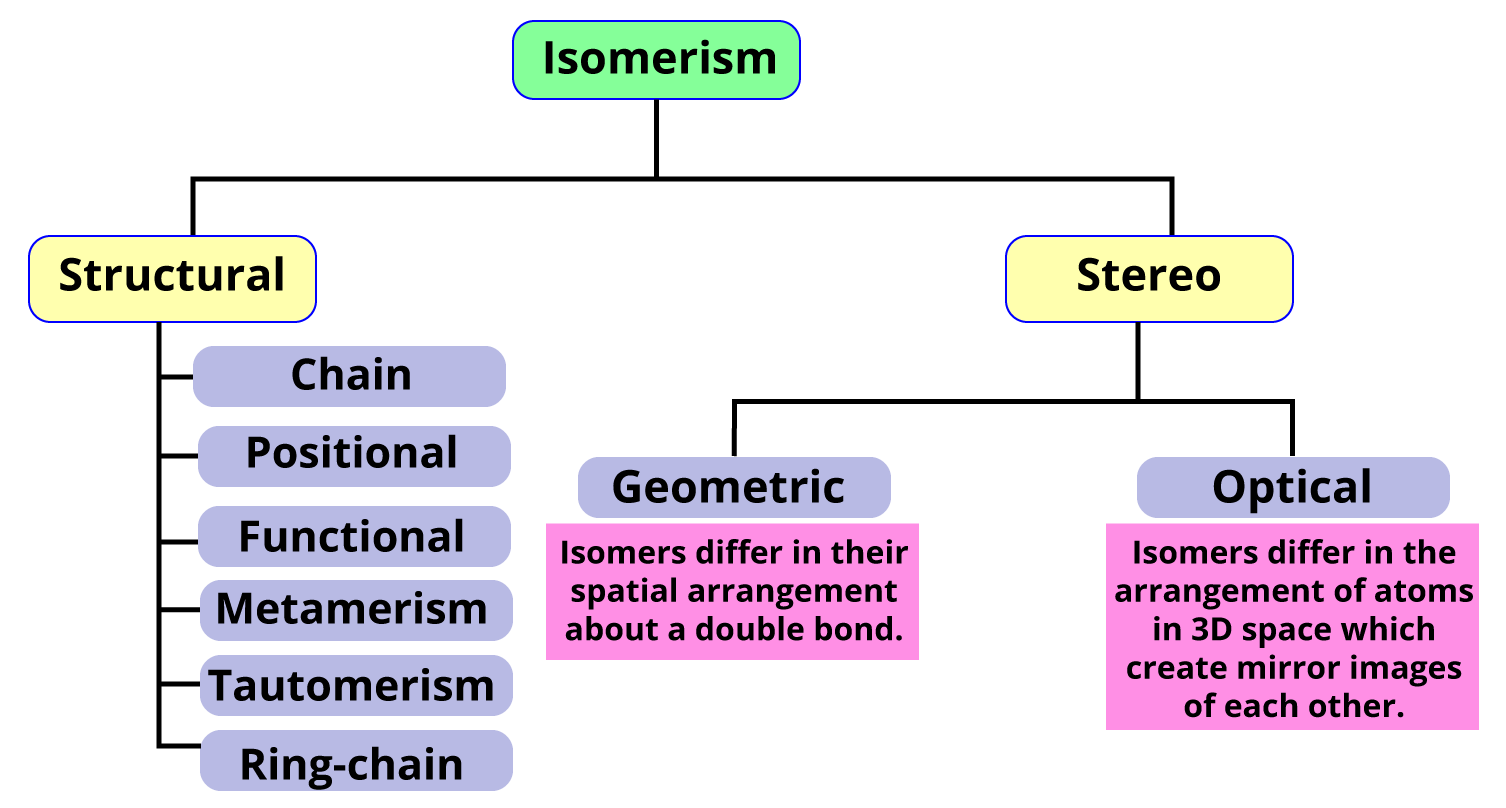
Types of Isomerism
In this article, we will discuss structural isomerism in detail. We will discuss its types and their examples as well as how to find the total number of structural isomers in a compound.
What Is a Structural Isomer?
The structural isomers have the same chemical formula but have their atoms totally rearranged in a different order. These are molecules with the same type of molecular formula but various connectivities based on the order in which they are assembled. It is also known as constitutional isomerism.
Types of structural Isomers
There are six types of structural isomers-
Chain Isomerism
Position Isomerism
Functional Isomerism
Metamerism
Tautomerism
Ring Chain isomerism
1. Chain Isomerism- Different configurations of carbon atoms result in both linear and branched chains, which give birth to chain isomerism. The linear and branched chains in the chain isomers share the same molecular formula.
For example, the molecular formula C4H12 makes two possible chain isomers, one of which is the linear chain isomer, i.e, n-butane and branched isomer, i.e, isobutane (or 2-methylpropane).
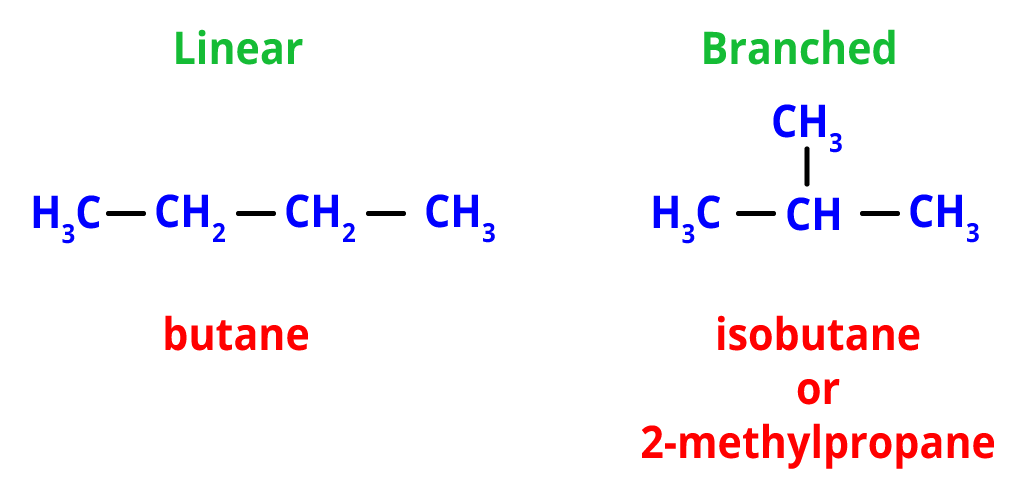
Chain Isomers of butane
2. Position Isomerism- Different positions of side chains, substituents, functional groups, double bonds, triple bonds, etc. on the parent chain are what lead to positional isomerism.
Positional isomers with the chemical formula C3H7Cl include propyl chloride and isopropyl chloride. These isomers arise due to differences in the position of the chloro group on the main chain.
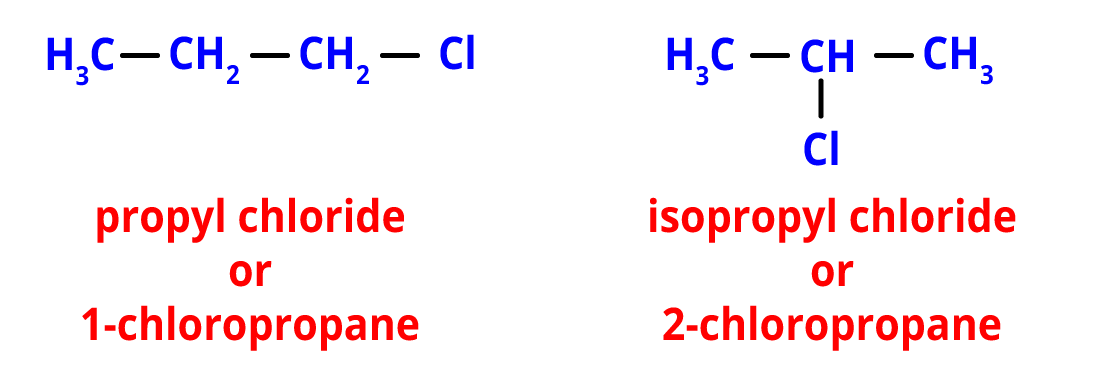
Position isomers of C3H7Cl
3. Functional Isomerism- The existence of various functional groups causes functional isomerism. The functional isomers have the same molecular formula but possess different functional groups.
For example, the functional isomer of ethyl alcohol is dimethyl ether. They both share the same chemical structure, C2H6O. They do, however, have various functional groups.
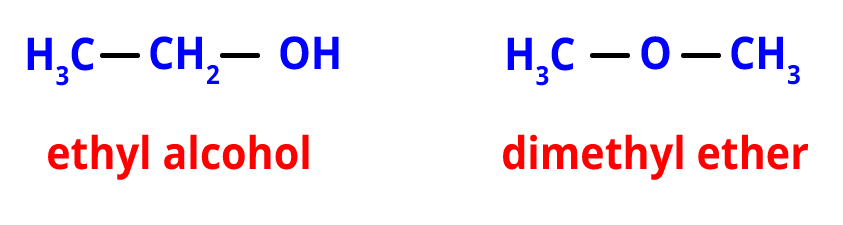
Functional isomers of C2H6O
4. Metamerism- When various alkyl groups are joined to the same functional group, metamerism results.
For example, the ether functional group is present in the following metamers. However, the type of alkyl groups that are joined to the oxygen atom in each one makes a difference.
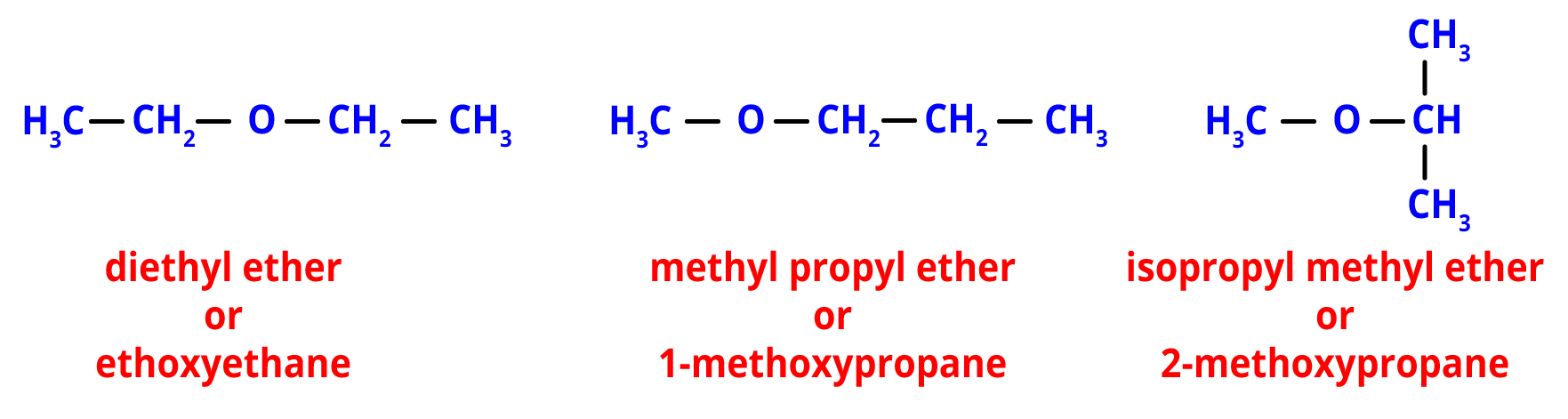
Metamers of C4H10O
5. Tautomerism- The dynamic equilibrium between two compounds having the same chemical formula is referred to as tautomerism. The tautomers live in dynamic equilibrium and have several functional groups.
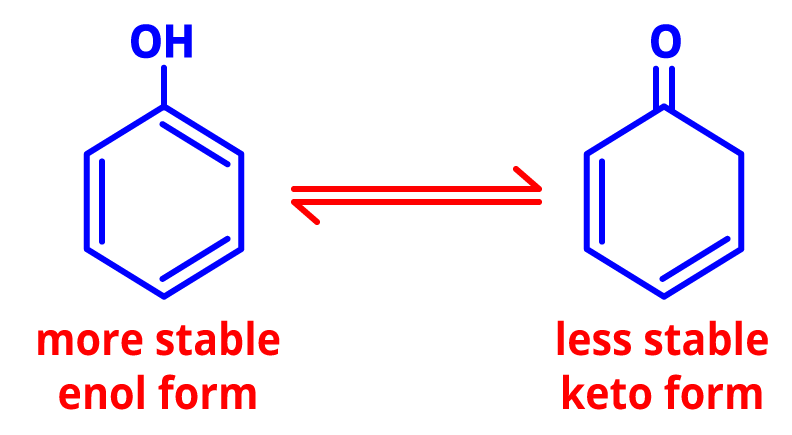
Tautomers of phenol
6. Ring chain isomerism- Ring chain isomers are substances with the same chemical formula but an open chain and cyclic structure.
Ring chain isomers include propene and cyclopropane, for instance.
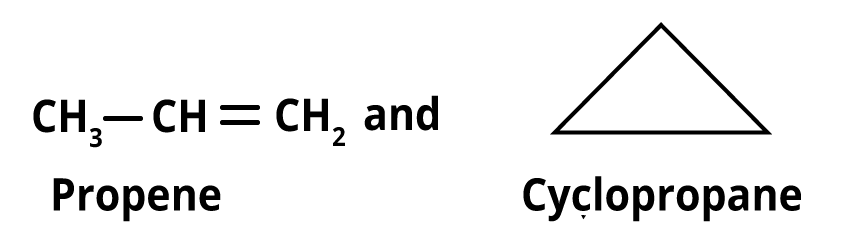
Ring chain isomerism of propene
How to Find Total Structural Isomers
The no of isomers formula can be calculated by using the formula-
$1+\sum_{i} \dfrac{n_{i}\left(v_{i}-2\right)}{2}$
Where, ni is the number of atoms and
vi= atoms valency
For example, let us calculate the total number of structural isomers is C8H10.
$\begin{align} &1+\sum_{i} \dfrac{n_{i}\left(v_{i}-2\right)}{2} \\ &1+\dfrac{8(4-2)}{2}+\dfrac{10(1-2)}{2} \\ &1+\dfrac{8 \times 2}{2}+\dfrac{10(-1)}{2} \\ &\dfrac{1+\dfrac{16}{2}-\dfrac{10}{2}}{2+16-10} \\ &\dfrac{8}{2}=4 \end{align}$
Therefore, there are 4 structural isomers in C8H10.
Structural isomerism of Hexane
Q. How many structural isomers are possible for C6H14?
Ans. The molecular formula of hexane is C6H14.
There are 5 structural isomers of hexane namely, n-hexane, 2-methylpentane, 3-methylpentane, 2,2-dimethylbutane, 2,3-dimethylbutane.
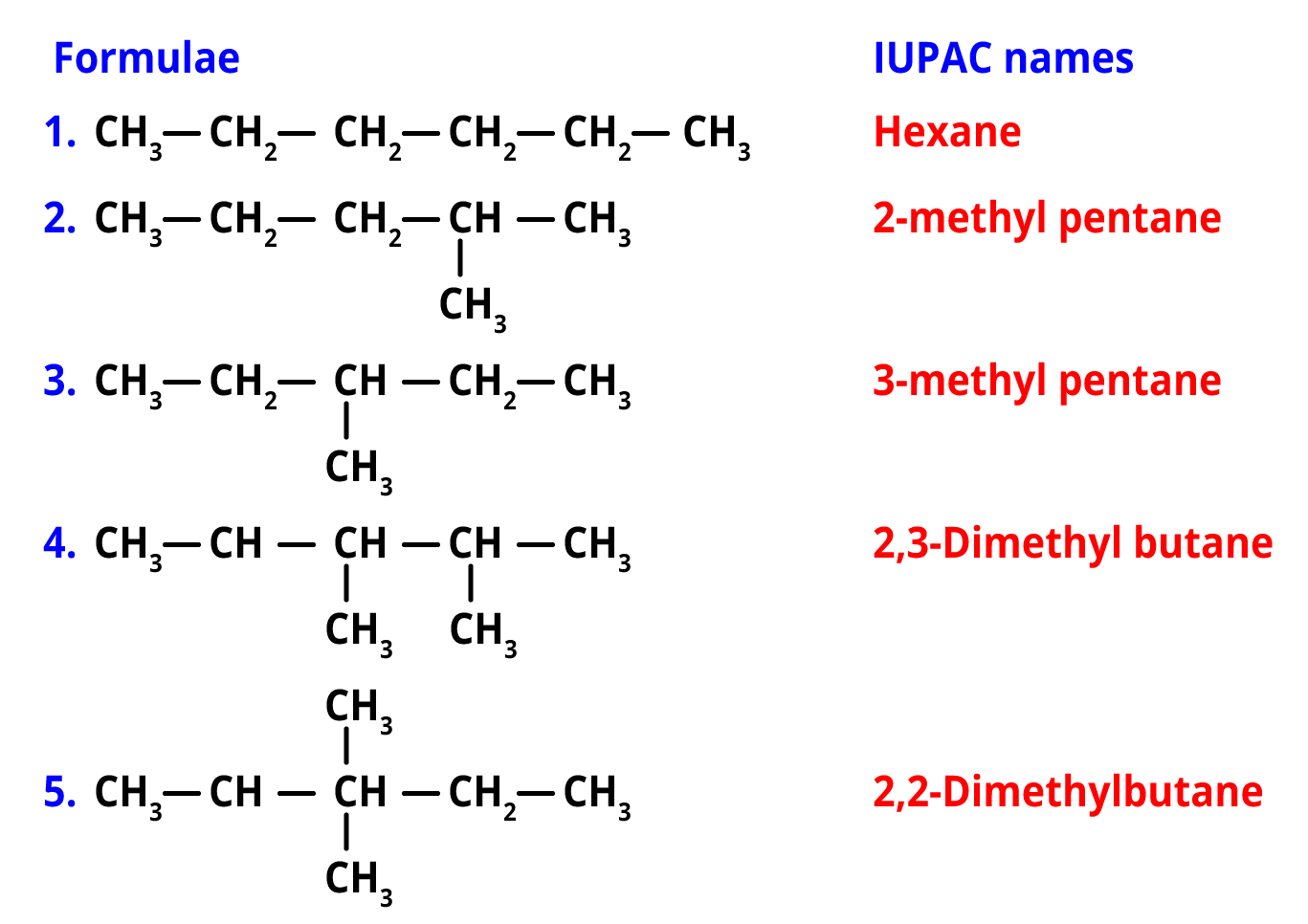
Structural isomers of hexane (C6H14)
Conclusion
Structural isomers and stereoisomers are the two main categories of isomers. Chain isomers, position isomers, and functional group isomers, metamers, tautomers, and ring chain isomers are the subgroups of structural isomers. Constitutional isomerism is the other name given to structural isomerism. In the molecules of these isomers, the atoms and functional groups are connected in various ways. Since distinct structural isomers might or might not include the same functional group, they are given unique IUPAC designations.
In this article we have discussed the isomers and their types i.e, structural isomerism and stereoisomerism, the structural isomers and their types, and the formula to calculate the structural isomers.
FAQs on Structural Isomers and Its Types for JEE Exam
1. What is stereoisomerism?
Stereoisomerism is also known as spatial isomerism or three dimensional isomerism. It is a type of isomerism used in stereochemistry in which molecules with the identical chemical structure and order of linked atoms (constitution) change in the three-dimensional spatial orientations of their atoms. Stereoisomers are divided into two types, namely configurational isomers (which occur in two different compounds and in which bond cleavage is required for bond breaking) and conformational isomers (which occur in identical compounds and do not require bond cleavage for interconversion). The configurational isomers are further subdivided into geometrical isomers and optical isomers.
2. What is the difference between enantiomers and diastereomers?
The difference between enantiomers and diastereomer is that
Enantiomers are stereoisomers that are not superimposable mirror images, but diastereomers are stereoisomers that are not superimposable and are not mirror images.
Diastereomers can be several molecules, but enantiomers are usually in pairs.
While diastereomers have different physical qualities from enantiomers, they both have the ability to rotate plane polarised light.
While the shapes of the molecules in enantiomers and diastereomers are similar, respectively.
Diastereomers can be separated by fractional distillation, chromatography, etc. while enantiomers cannot be separated by crystallisation.
3. What is the difference between structural isomers and stereoisomers?
The difference between structural isomers and stereoisomers is that-
While they both have the same chemical formula, structural isomers have different atomic arrangements, whereas stereoisomers have the same chemical formula and atomic arrangements but different spatial arrangements.
Stereoisomers have the same arrangement of atoms as structural isomers, whereas the latter have different atom configurations.
While stereoisomers need the presence of chiral atoms, structural isomers lack chiral carbon atoms.
Compared to stereoisomers, structural isomers have physical and chemical properties that are distinct from each other.
























