




How to Identify the Most Stable Resonance Structure with Examples
Understanding the stability of resonance structures is crucial for mastering organic chemistry in JEE. Resonance structures depict electron delocalisation within a molecule, affecting molecular stability, reactivity, and exam-focused problem solving. This helps determine which canonical form most influences the actual molecular hybrid, and thus aids in predicting correct MCQ answers. Accurate recognition of more stable contributors is vital for JEE success.
What Are Resonance Structures?
A resonance structure refers to one of two or more valid Lewis representations for a molecule or ion with delocalised electrons. These contributor forms differ only in the positions of electrons, not atoms. For polyatomic ions and molecules like phenol, a single Lewis structure cannot capture all bonding features, so the real structure—the resonance hybrid—represents an average of its canonical forms. This concept is central not just in the General Organic Chemistry, but also in aromatic compounds and reaction mechanisms.
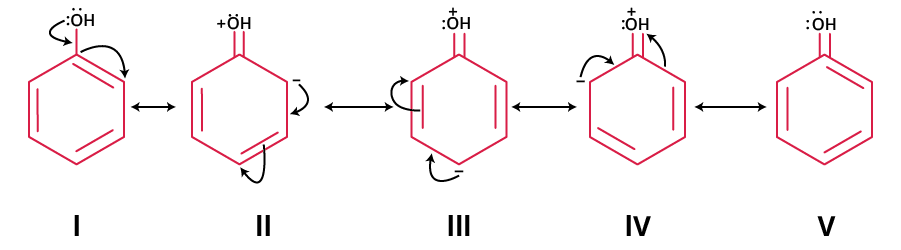
Resonance helps explain fractional bonds or charges as found in benzene, nitrate, or carbonate ions. Always ensure that only electrons (not atomic positions) shift between forms. For example, phenol exhibits several resonance contributors to stabilise its structure due to pi-electron delocalisation.
Stability of Resonance Structures — Key Principle
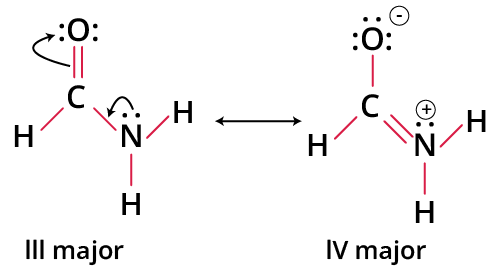
The stability of resonance structures depends on several factors that distinguish more influential canonical forms in the resonance hybrid. The most stable resonance structure has the lowest energy and contributes the most to the true electronic structure. Unstable contributors, higher in energy, play a minor role. All valid forms taken together yield the resonance hybrid, reflecting delocalized pi electrons, reduced charge separation, and maximised covalent character. JEE MCQs often test recognition of these main contributors.
Rules for Estimating Stability of Resonance Structures
- The most stable structure completes the octet for all involved atoms.
- Structures with the least number of formal charges or no charges are more stable.
- A negative charge on a more electronegative atom (e.g., O, N) and a positive charge on a more electropositive atom (e.g., C, metal) enhance stability.
- Stability increases with maximum covalent bonds (more bond pairs).
- Less charge separation (minimum distance between positive and negative charges) leads to higher stability.
An easy memory trick: use the order—octet completion > minimal charges > charge on suitable atom > less charge separation > more bonds.
- Structures violating the octet (except for exceptions like BF3 or radicals) are less stable.
- Resonance contributors with extensive charge separation, or with charges on wrong atoms, are never the main contributors.
Factors Controlling Resonance Stability — Clarifying Table
| Stability Factor | Structure Favoured |
|---|---|
| Complete Octet | Always more stable (C, O, N with full octet) |
| Minimal Formal Charges | No/more neutral structures preferred |
| Charge — Atom Suitability | Negative on O/N, Positive on C/less EN atom |
| Max. Covalent Bonds | Structures with more bonds preferred |
| Less Charge Separation | Opposite charges closer = more stability |
In mesomeric effect or resonance effect questions, always check if the octet is fulfilled before considering charges or bonds.
Real Examples: Application on Resonance Contributors
Let’s analyse classic examples encountered in JEE:
- Phenol: Lone pairs on oxygen delocalise into the ring, creating negative charge at ortho and para positions. Forms where all atoms have a full octet and negative charge on oxygen are most stable contributors.
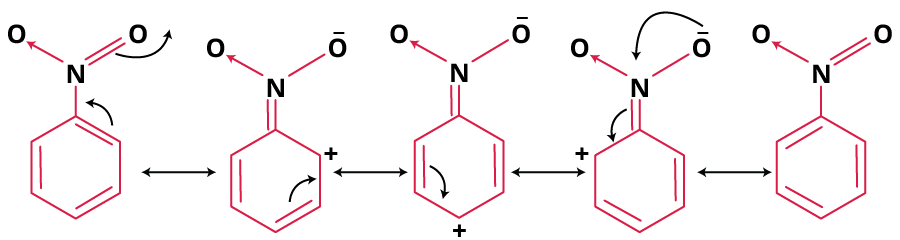
- Nitrobenzene: Resonance pushes positive charge to meta region. Main contributors have octet completion and localised negative charge on oxygen.
- Chlorobenzene: Chlorine’s lone pair resonates with benzene, increasing ortho and para electron density, but main stabilization comes from forms keeping octet intact for all atoms.
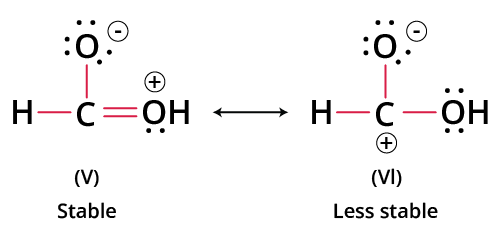
- Acetic Acid: The structure with charge separation between oxygen and carbon is less stable; neutral forms with completed octet are primary contributors.
Why Resonance Hybrids Are More Stable
The resonance hybrid is a single “real” electronic arrangement resulting from combining all possible contributors. Delocalisation reduces the molecule’s potential energy, making resonance hybrids more stable than any individual resonance contributor. The more contributors fulfil the key rules, the more stable (and influential) the hybrid becomes. Resonance energy quantifies this stabilisation.
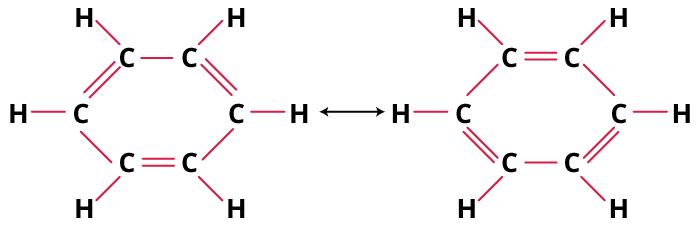
Stepwise Method to Identify the Most Stable Structure
- Draw all valid resonance contributors—move only electrons, not atoms.
- Check octet completion for each atom in every form.
- Count formal charges; prioritise structures with the fewest.
- Place negative charge on more electronegative elements (O, N).
- Minimize charge separation; keep charges close.
- Compare all, pick the one(s) satisfying most criteria as the primary contributor.
For more practice, see how to draw resonance structures and test yourself with additional examples from previous year JEE Main papers.
Resonance Problems: JEE Example
Example: Between the following resonance forms of the acetate ion, which is more stable?
- Structure I: Both oxygens have a -0.5 charge (resonance hybrid)
- Structure II: C double bonded to one O-, other O has a single bond (charge delocalised)
- Structure III: C+ bonded to O- (charge entirely separated)
Structure II is more stable than III, because all atoms in II have completed octets and charges are on appropriate atoms, matching our resonance stability criteria. III, with extensive charge separation, is least stable. The hybrid (I) depicts equal charge delocalisation and is overall the lowest in energy.
Common Pitfalls and Exam Tips
- Never shift or break atomic bonds; move only pi electrons or lone pairs.
- Never count resonance forms with violated octets as primary—except for accepted exceptions (radicals or deficient elements).
- Resonance is valid only for conjugated, alternating double-single bondsystems or when lone pairs are adjacent to multiple bonds.
- In MCQs, prefer canonical forms obeying all five rules over those with incomplete octet, excess charge, or wrong charge placement.
A quick scan using these pointers ensures rapid and accurate answer selection for JEE Main and Advanced.
Summary: Predicting Stability of Resonance Structures in JEE
In summary, the stability of resonance structures increases with complete octet, minimum formal charges, correct charge placement, high covalent character, and less charge separation. The most stable contributor—often neutral with full octet—dominates the resonance hybrid and influences reactivity. Always draw all possible canonical forms before judging stability for MCQs. For more on related topics, try these: Resonance Effect, Mesomeric Effect, Inductive Effect vs. Resonance Effect, and more at Vedantu. This foundation is essential for cracking tougher organic questions and understanding real-life chemical behaviour.
FAQs on Stability of Resonance Structures in Organic Chemistry
1. What factors affect the stability of resonance structures?
The stability of resonance structures depends on several key factors: For exam questions, always look for these main points:
- Complete octet: Structures where all atoms, especially second-period elements, have a full octet are more stable.
- Minimum formal charges: Less overall charge separation increases stability.
- Negative charge on more electronegative atoms (like O or N) and positive charge on less electronegative atoms (like C).
- Maximum number of covalent bonds (more bond pairs, fewer charge separations).
- Delocalization: More delocalization of electrons increases resonance stability.
2. What makes resonance structures more stable?
The most stable resonance structures minimize formal charges and maximize octet satisfaction. Stability increases when:
- All atoms achieve a full octet, where applicable.
- Negative charges are on electronegative atoms.
- There is minimum charge separation.
- More covalent bonding and delocalization occurs.
3. How do you determine the most stable resonance structure?
To determine the most stable resonance structure, apply these rules:
- Check if the structure follows the octet rule for all atoms.
- Count and compare formal charges; fewer is better.
- Assign negative charges to the most electronegative atoms.
- Avoid excessive charge separation.
- Prefer structures with more bonds and delocalization.
4. What is a resonance hybrid structure?
A resonance hybrid is the actual structure of a molecule, combining all valid resonance forms. It does not exactly match any single resonance structure but represents the weighted average of contributors.
- Depicted by dashed lines for partial bonds and partial charges.
- Shows delocalization of electrons across the molecule.
- Is always more stable than any individual resonance form.
5. How does resonance stability impact reactivity in organic chemistry?
Resonance stability affects how easily molecules take part in chemical reactions. Stable resonance structures can:
- Reduce a molecule's tendency to react (e.g., benzene's low reactivity due to stable resonance hybrid).
- Stabilize reactive intermediates like carbocations or carbanions.
- Alter preferred reaction mechanisms in organic synthesis.
6. Are resonance hybrids more stable than individual structures?
Yes, a resonance hybrid is always more stable than any individual resonance structure. This increased stability comes from the delocalisation of electrons and lower overall energy. In exams, remember that resonance always increases stability compared to individual canonical forms.
7. Why are some resonance structures more stable than others?
Some resonance structures are more stable because they minimize formal charges and follow the octet rule. Stability also increases if negative charges reside on electronegative atoms and the structure has less charge separation.
These features should be prioritized when selecting the most stable resonance form in questions.
8. Can resonance involve atoms that don’t have a complete octet?
Yes, but such resonance structures are less stable and contribute less to the hybrid. Structures with incomplete octets (usually on elements like carbon, nitrogen, or oxygen) are generally considered only when no alternatives exist or in special cases (e.g., carbocations). For exams, focus on forms with a complete octet for maximum stability.
9. Is every drawn structure a valid resonance contributor?
No, not all drawn structures are valid resonance contributors. A valid resonance structure must:
- Preserve the positions of nuclei; only electrons move.
- Have a correct number of electrons for each atom (especially follow the octet rule).
- Show allowable movement of electrons, typically using pi bonds or lone pairs.
Structures violating these rules are not counted in resonance hybrids during exams.
10. Do resonance rules differ for carbocations, carbanions, and free radicals?
Basic resonance rules remain similar, but application varies for carbocations, carbanions, and free radicals. For carbocations:
- Stability depends on delocalization of positive charge.
- Stability increases with delocalized negative charge on electronegative atoms.
- Resonance can delocalize the unpaired electron, enhancing stability.
11. How do mistakes in assigning formal charges affect resonance stability predictions?
Incorrect formal charges lead to wrong identification of the most stable resonance form. These mistakes can result in:
- Violating the octet rule.
- Assigning charges to less suitable atoms (e.g., negative on carbon over oxygen).
- Favoring less stable structures in exam answers.

































