




How to Identify Square Planar Complexes: Rules, Examples & Key Features
A strong understanding of the square planar complex is essential for mastering coordination chemistry, especially for JEE Main. In these compounds, four ligands arrange themselves around a central transition metal ion in a single plane, with 90° bond angles, forming a distinct square shape. Recognizing their structure, hybridisation, and d-orbital splitting patterns helps solve conceptual and application-based questions typical in entrance exams. The square planar geometry also connects directly with electronic configuration, especially for d8 metal ions such as Ni2+, Pd2+, and Pt2+.
What is a Square Planar Complex?
Square planar complexes are a type of coordination compound in which four ligands are symmetrically positioned at the corners of a square, all in one plane, around a central metal ion. This arrangement yields a bond angle of 90° between adjacent ligand-metal-ligand directions. Square planar geometry is particularly common for transition metals with a d8 configuration, where crystal field effects and hybridization lower the energy for this specific shape as compared to tetrahedral alternatives.
How to Identify a Square Planar Complex
To determine if a coordination compound adopts square planar geometry, follow these evidence-based checks:
- Check the central metal’s electronic configuration: d8 transition metals (like Ni2+, Pd2+, Pt2+) are prime candidates.
- Look for strong-field ligands (e.g., CN-, NH3, Cl-) which can induce pairing of electrons in the d orbitals, making square planar more stable than tetrahedral.
- For coordination number 4, compare with possible tetrahedral geometry.
- If hybridization is confirmed as dsp2, expect square planar structure for the complex ion.
- Empirical evidence like diamagnetism (all electrons paired) often supports square planar geometry.
Key Examples and Formulas: Square Planar Complexes
Learning core examples helps with quick identification and recall in exams. Typical square planar complexes are listed below, with formula and center metal’s configuration highlighted for JEE:
| Complex Formula | Central Metal | d-Electron Count | Geometry |
|---|---|---|---|
| [Ni(CN)4]2− | Ni2+ | d8 | Square Planar |
| [Pt(NH3)2Cl2] | Pt2+ | d8 | Square Planar |
| [PdCl4]2− | Pd2+ | d8 | Square Planar |
| [Cu(NH3)4]2+ | Cu2+ | d9 | Can be Square Planar |
The classic example for exam purposes is [Ni(CN)4]2−, featuring strong-field cyanide ligands and a diamagnetic, square planar structure. The square planar geometry of [NiCN4]2- is frequently asked in objective-type questions.
![Square planar geometry showing arrangement in [Ni(CN)4]2- complex ion for JEE Main](https://www.vedantu.com/seo/content-images/7579aaa0-854d-4f79-91ca-50b4eecbef81.png)
Hybridisation and Bonding in Square Planar Complexes
The hybridization involved in a square planar complex is dsp2. Here, one d orbital (typically dx2-y2), one s, and two p orbitals combine to produce four equivalent hybrid orbitals lying in a plane. This arrangement allows the metal to accept electron pairs from four ligands, producing strong bonding and the square configuration.
- dsp2 hybridization: 1 d (usually dx2-y2), 1 s, 2 p orbitals (px, py).
- The central metal must have at least one empty d orbital, which is often achieved by pairing up d electrons through ligand field interaction.
- This hybridisation is mainly seen in d8 or occasionally d9 ions with strong field ligands.
The formation of square planar hybridization [Ni(CN)4]2− complex, involving 3d4sp2 hybridization of Ni2+ ion orbitals, illustrates the process for JEE questions.
![Hybridization and electronic configuration diagram for square planar [Ni(CN)4]2- in JEE coordination compounds](https://www.vedantu.com/seo/content-images/9fdaaba1-64db-47d8-884a-e0bccdc50dd6.png)
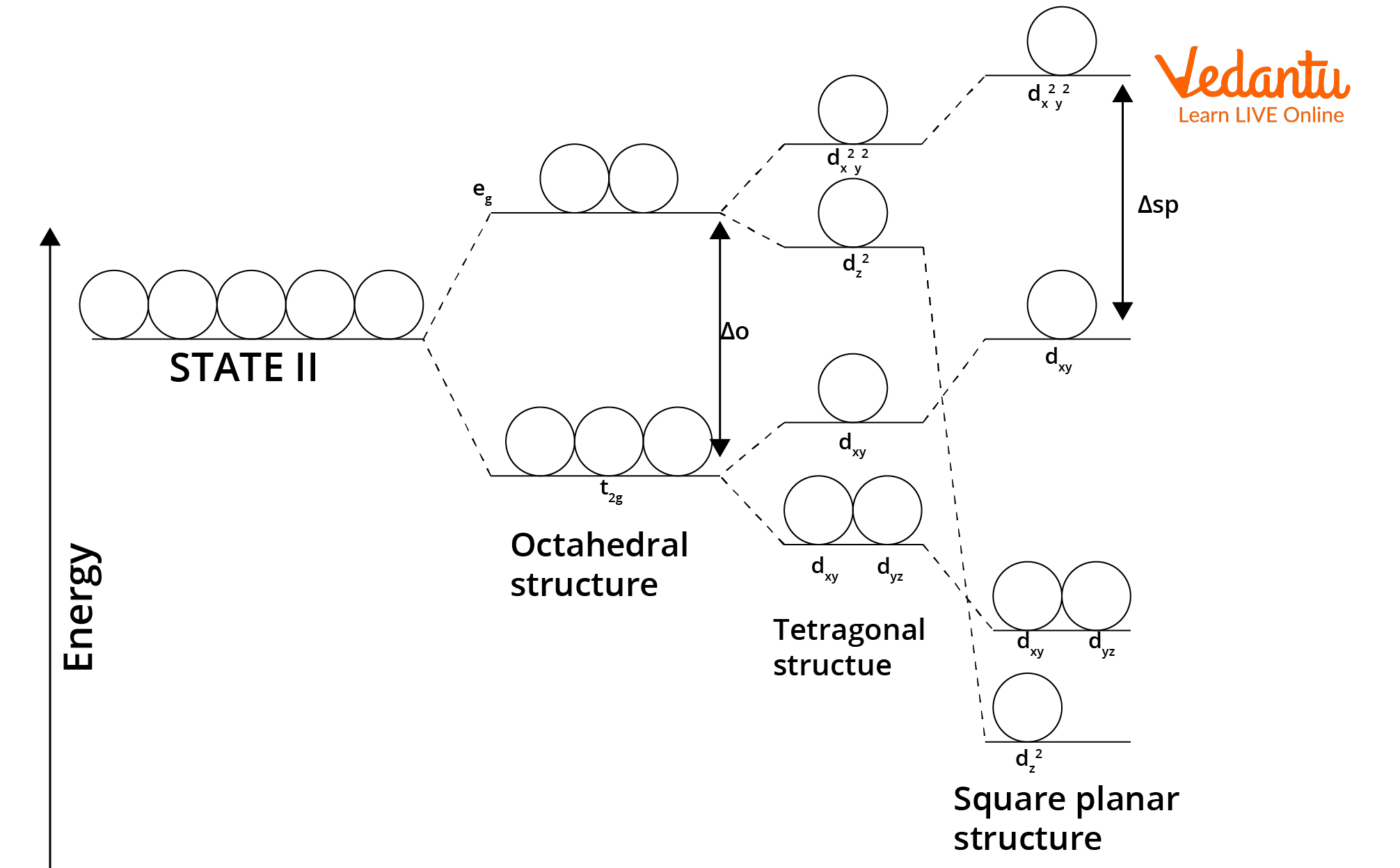
Crystal Field Splitting in Square Planar Complexes
In square planar complexes, the five d orbitals experience different repulsions due to the arrangement of the four ligands in a plane, causing them to split into energy levels. The dx2-y2 orbital is most destabilized because it points directly at the ligands, whereas dz2, dxy, dxz, and dyz are less affected. The splitting energy (Δsp) is larger than in tetrahedral fields and comparable to the octahedral field with removed axial ligands.
- Order of increasing energy: dxz, dyz < dz2 < dxy < dx2-y2.
- dx2-y2 orbital lies in the same plane as the ligands and thus is highest in energy.
- Complete pairing of electrons is favored in strong-field, square planar cases, often leading to diamagnetic behavior.
Crystal field splitting in the square planar complexes is often visualized in questions requiring orbital energy sequence or magnetic property prediction.
Square Planar vs Tetrahedral Geometry: Key Differences
A frequent JEE Main question type is to contrast square planar and tetrahedral geometries for coordination compounds with coordination number 4. The table below summarizes their core differences for quick revision.
| Aspect | Square Planar | Tetrahedral |
|---|---|---|
| Ligand Arrangement | 4 in one plane, corners of a square | 4 at corners of a tetrahedron, not in one plane |
| Bond Angles | 90° | 109.5° |
| Hybridization | dsp2 | sp3 |
| Field Preference | Favored by strong field ligands, d8 metal ions | Favored by weak field ligands, variable d-count |
| Example | [Ni(CN)4]2− | [NiCl4]2− |
Common Pitfalls and Exam-Style Applications
JEE aspirants sometimes mistakenly assign square planar geometry to all d8 or 4-coordinate metal ions, overlooking electronic factors or ligand strength. Always confirm strong-field ligand presence and check for dsp2 hybridization. Application problems may require you to:
- Predict geometry based on ligands: [Ni(CN)4]2− is square planar, [NiCl4]2− is tetrahedral due to Cl- being a weak field ligand.
- Use magnetic properties: Diamagnetic 4-coordinate d8 complexes are often square planar.
- Draw d-orbital splitting diagrams—and use them to justify shapes.
- Apply valence bond theory or crystal field theory to deduce hybridization.
TIP: Always check if the central ion is a late transition metal (like Ni, Pd, Pt) and the complex is diamagnetic. If so, suspect square planar geometry for the compound.
Summary and Quick Revision Points
- Square planar complexes have four ligands placed at 90° around a central metal in a plane.
- Favored by d8 ions like Ni2+, Pd2+, Pt2+ with strong-field ligands.
- dsp2 hybridization is characteristic of square planar geometry.
- Crystal field splitting puts dx2-y2 highest in energy for these complexes.
- Compare carefully with tetrahedral: sp3 hybridization and weak field ligands indicate non-planar shapes.
- Classic example: [Ni(CN)4]2− is square planar and diamagnetic.
To dive deeper, refer to Coordination Compounds, Hybridization, Coordination Compounds Mock Test, and Chemical Bonding and Molecular Structure on Vedantu for more solved questions and additional conceptual clarity. Mastering the concepts of square planar complexes will strengthen your confidence in d- and f-block elements and complex ion geometry. This understanding is vital for high scores in the JEE Main Chemistry paper.
FAQs on Square Planar Complex in Coordination Chemistry
1. What is a square planar complex?
A square planar complex is a type of coordination compound in which a central metal ion is surrounded by four ligands placed at the corners of a square in the same plane, with bond angles of 90°.
Key points:
- Common in d8 metal ions (e.g., Ni2+, Pd2+, Pt2+).
- Characteristic bond angle is 90°.
- Major examples include [Ni(CN)4]2− and [Pt(NH3)2Cl2] (cisplatin).
2. How to know if a complex is square planar?
You can identify a square planar complex by analyzing the metal's electronic configuration, the type of ligands, and hybridization.
Consider the following:
- Look for d8 metal ions (like Ni2+, Pd2+, Pt2+).
- The complex should have four ligands and exhibit dsp2 hybridization.
- Presence of strong field ligands promotes square planar geometry.
- If the molecule is planar with 90° angles between ligands, it is likely square planar.
3. What is an example of square planar structure?
Classic examples of square planar complexes include:
- [Ni(CN)4]2−
- [Pt(NH3)2Cl2] (cisplatin)
- [PdCl4]2−
4. Are all d8 complexes square planar?
Not all d8 complexes are square planar. The geometry depends on the metal, ligand strength, and crystal field effects.
Important notes:
- Nickel(II), Palladium(II), and Platinum(II) often form square planar complexes with strong field ligands.
- However, with weak field ligands, they may adopt a tetrahedral geometry instead.
5. What is the hybridization of square planar complexes?
Square planar complexes typically exhibit dsp2 hybridization.
Key details:
- Hybridization involves one d, one s, and two p orbitals from the central atom.
- This hybridization allows four ligands to arrange themselves in a single plane at 90° bond angles.
6. What is the crystal field splitting in square planar complexes?
In square planar complexes, the crystal field splitting of the d-orbitals is significant and different from octahedral geometry.
The order of d-orbital energies (from lowest to highest) is:
- dz2
- dxz and dyz
- dxy
- dx2-y2 (highest)
7. What is the difference between square planar and tetrahedral complexes?
Square planar and tetrahedral complexes differ in geometry, hybridization, and electronic properties.
Key differences:
- Square planar: Four ligands in a single plane, bond angles 90°, dsp2 hybridization, often observed with d8 metals.
- Tetrahedral: Four ligands positioned at corners of a tetrahedron, bond angles 109.5°, sp3 hybridization, commonly seen with d10 and sometimes d8 metals with weak field ligands.
8. What are some common examples of square planar complexes?
Common square planar complexes include:
- [Ni(CN)4]2−
- [PtCl4]2−
- [Pd(NH3)4]2+
- [AuCl4]
9. How do strong field ligands affect the geometry choice in d8 complexes?
Strong field ligands (like CN−, CO) favor square planar geometry in d8 metal ions.
This is because:
- They increase crystal field splitting energy.
- Stabilize the dsp2 hybridization.
- Promote low spin configurations and planar arrangement of ligands.
10. Is dsp2 hybridization involved in square planar complexes?
Yes, square planar complexes involve dsp2 hybridization.
This hybridization allows the central metal ion to arrange four ligands at 90° angles within the same plane, a hallmark of square planar geometry in coordination compounds.























